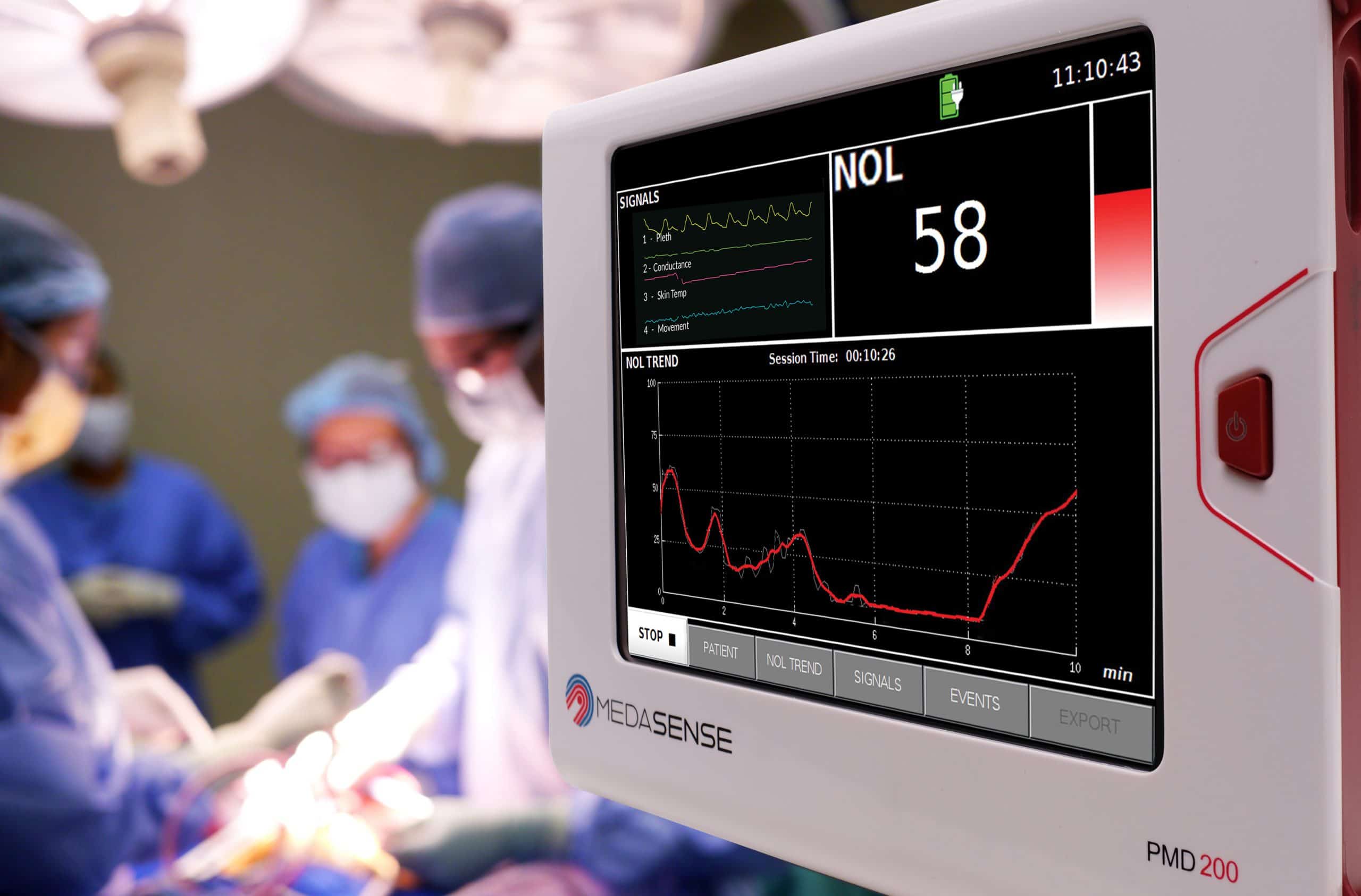

Medasense has announced that its NOL® pain response monitoring device (PMD-200™) has been approved for marketing in Brazil by ANVISA, the Brazilian health regulatory agency.
An early user of the NOL in Brazil, Dr. José Luiz Gomes do Amaral, Professor and Chairman of the Department of Anesthesiology, Pain & Critical Care at Escola Paulista de Medicina Hospital São Paulo, stated: “The introduction of NOL monitoring in Brazil is an exciting development for me and my colleagues, as I believe this might be the breakthrough technology we have all been looking for to help us provide better pain medication treatment.”
Karine Moriya, CEO of J.G. Moriya, Medasense commercial partner in Brazil, commented: “Our network of influential surgeons and anesthesiologists should allow us to bring this much-needed technology to Brazil’s advanced surgical facilities and we expect to perform the first NOL-guided surgeries in the coming weeks.”
more recommended stories
 Texas Medical Board Releases Abortion Training for Physicians
Texas Medical Board Releases Abortion Training for PhysiciansKey Takeaways Texas Medical Board has.
 Safer Allogeneic Stem Cell Transplants with Treg Therapy
Safer Allogeneic Stem Cell Transplants with Treg TherapyA new preclinical study from the.
 Autoimmune Disorders: ADA2 as a Therapeutic Target
Autoimmune Disorders: ADA2 as a Therapeutic TargetAdenosine deaminase 2 (ADA2) has emerged.
 Kaempferol: A Breakthrough in Allergy Management
Kaempferol: A Breakthrough in Allergy ManagementKaempferol, a dietary flavonoid found in.
 Early Milk Cereal Drinks May Spur Infant Weight Gain
Early Milk Cereal Drinks May Spur Infant Weight GainNew research published in Acta Paediatrica.
 TaVNS: A Breakthrough for Chronic Insomnia Treatment
TaVNS: A Breakthrough for Chronic Insomnia TreatmentA recent study conducted by the.
 First-of-Its-Kind Gene-Edited Pig Kidney: Towana’s New Life
First-of-Its-Kind Gene-Edited Pig Kidney: Towana’s New LifeSurgeons at NYU Langone Health have.
 Just-in-Time Training Improves Success & Patient Safety
Just-in-Time Training Improves Success & Patient SafetyA study published in The BMJ.
 ChatGPT Excels in Medical Summaries, Lacks Field-Specific Relevance
ChatGPT Excels in Medical Summaries, Lacks Field-Specific RelevanceIn a recent study published in.
 Study finds automated decision minimizes high-risk medicine combinations in ICU patients
Study finds automated decision minimizes high-risk medicine combinations in ICU patientsA multicenter study coordinated by Amsterdam.

Leave a Comment