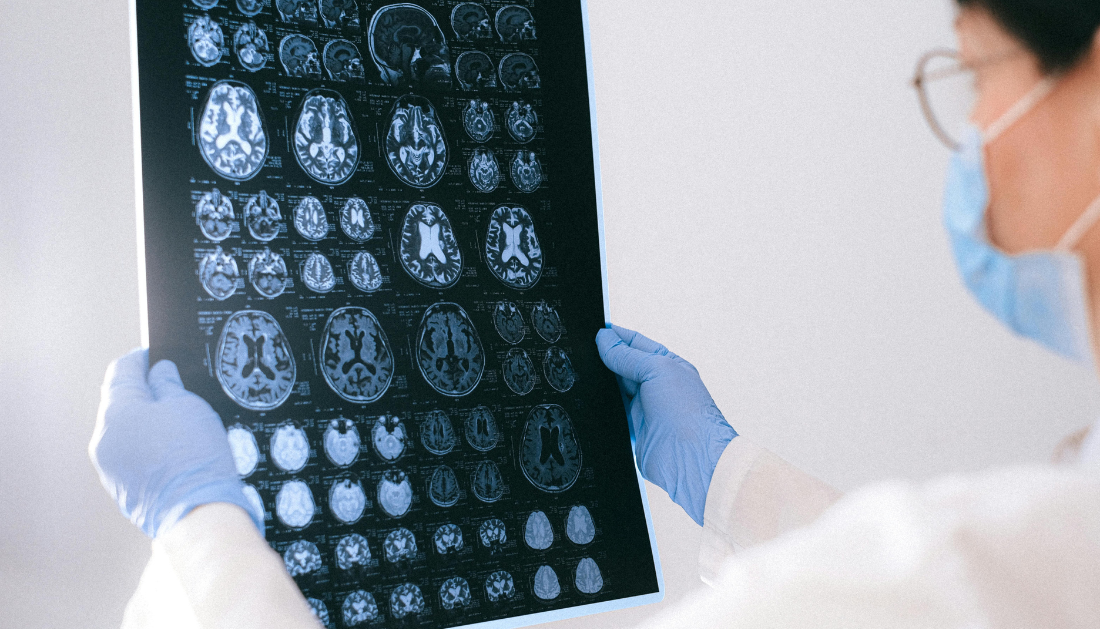

The International Progressive MS Alliance has launched the MS Clinical and Imaging Data Resource (CIDR), a major initiative designed to fast-track treatment development for progressive multiple sclerosis. With over 72,000 anonymized MRI scans and clinical data from more than 13,500 individuals with MS, this €685 million resource offers the academic and clinical research community unprecedented access to harmonized datasets.
Explore All Neurology CME/CE Conferences
Why This Progressive MS Research Matters for Clinicians and Researchers
For neurologists, radiologists, and healthcare professionals managing MS, CIDR provides data from 200,000 clinical visits across multiple international trials. The integration of imaging and clinical information allows investigators to identify early disease progression signals, evaluate treatment responses, and improve trial efficiency.
“There are currently no fully validated biomarkers to predict disease progression in progressive MS. This is a hurdle because large phase III trials might cost up to hundreds of millions of dollars each, so if they don’t work, it’s a big loss for the company and society.”
Prof. Douglas Arnold, McGill University
Currently, most therapies target relapsing MS, leaving progressive MS an unmet clinical need. By making harmonized trial data available, the Alliance aims to enable natural history modeling, simulation studies, and biomarker discovery. With the inclusion of AI and machine learning approaches, researchers can test predictive algorithms to identify patients who may respond better to experimental therapies, potentially shortening the length and cost of future trials.
Clinical Implications for Healthcare Professionals
For clinicians, this initiative could mean earlier identification of progression, improved patient stratification, and more efficient therapeutic options for progressive MS. The ability to access large-scale imaging and clinical datasets enables better comparison of patient outcomes, refinement of diagnostic markers, and collaboration across global centers.
AI-assisted tools embedded in CIDR hold promise for reducing clinical trial sizes without compromising reliability, ensuring that effective therapies reach patients sooner. For nurses and allied HCPs, the harmonization of patient data also emphasizes the importance of consistent documentation and care coordination, highlighting the collective role of the healthcare community in progressive MS management.
As applications open in late 2025, this resource represents a step forward in collaborative MS research and a practical asset for healthcare teams worldwide seeking to improve patient care.
For More information:
more recommended stories
 Phage Therapy Study Reveals RNA-Based Infection Control
Phage Therapy Study Reveals RNA-Based Infection ControlKey Takeaways (Quick Summary) Researchers uncovered.
 Safer Allogeneic Stem Cell Transplants with Treg Therapy
Safer Allogeneic Stem Cell Transplants with Treg TherapyA new preclinical study from the.
 AI in Emergency Medicine and Clinician Decision Accuracy
AI in Emergency Medicine and Clinician Decision AccuracyEmergency teams rely on rapid, accurate.
 Innovative AI Boosts Epilepsy Seizure Prediction by 44%
Innovative AI Boosts Epilepsy Seizure Prediction by 44%Transforming Seizure Prediction in Epilepsy Seizure.
 Hypnosis Boosts NIV Tolerance in Respiratory Failure
Hypnosis Boosts NIV Tolerance in Respiratory FailureA New Approach: Hypnosis Improves NIV.
 Bee-Sting Microneedle Patch for Painless Drug Delivery
Bee-Sting Microneedle Patch for Painless Drug DeliveryMicroneedle Patch: A Pain-Free Alternative for.
 AI Reshapes Anticoagulation in Atrial Fibrillation Care
AI Reshapes Anticoagulation in Atrial Fibrillation CareUnderstanding the Challenge of Atrial Fibrillation.
 Hemoglobin as Brain Antioxidant in Neurodegenerative Disease
Hemoglobin as Brain Antioxidant in Neurodegenerative DiseaseUncovering the Brain’s Own Defense Against.
 AI Diabetes Risk Detection: Early T2D Prediction
AI Diabetes Risk Detection: Early T2D PredictionA new frontier in early diabetes.
 Cancer Cells Learn to Self-Report: A New Frontier in Immunotherapy
Cancer Cells Learn to Self-Report: A New Frontier in ImmunotherapyHow a Drug Complex Enables Immune.

Leave a Comment