
CAR T-cell therapies have transformed the way some cancers are treated, but they have a major drawback in that many patients, even those whose cancer goes into complete remission, eventually have a return. Researchers at the Dana-Farber Cancer Institute have published a new paper that details a method that might solve that issue.
The strategy, which is detailed in a research that was posted online today in the journal Nature Biotechnology, allows CAR T cells to stay in combat mode until all tumor cells have been eradicated by encouraging them to be more active and stay in the body longer. The method also causes CAR T cells to develop a memory of the cancer cell, enabling them to reactivate in the event that the disease returns. This is known as a CAR-Enhancer (CAR-E) therapeutic platform.
Clinical trials of this method in human patients have been made possible by the CAR-Enhancer treatment’s ability to eradicate all tumor cells in research conducted on patient-derived laboratory cancer cell lines and other studies. The first experiment is anticipated to begin soon, according to the researchers.
“CAR T-cell therapies have been a breakthrough treatment for B-cell hematologic cancers such as B-cell leukemias and lymphomas and multiple myeloma,” says the study’s senior author, Mohammad Rashidian, PhD, of Dana-Farber.
“In myeloma, for example, virtually 100 percent of patients have an excellent response to CAR T-cell therapies initially, but almost all relapse, half of them within one to two years of treatment. Relapse coincides with the disappearance of CAR T-cells in the bloodstream.”- Mohammad Rashidian, Dana-Farber Cancer Institute
“Most of the research to address this challenge has focused on re-engineering the CAR T cell itself – for example, by introducing or eliminating genes to keep the cell active for longer,” he continues. “While these approaches hold great promise, they have yet to show much effectiveness in the clinic. We decided to come at the problem from a completely different perspective.”
Rather of attempting to modify the internal mechanisms of CAR T cells, Rashidian and associates devised an external strategy that functions by bringing a chemical that prolongs the cells’ lifespan and stimulates memory formation right to their doorstep. This is being accomplished with a vehicle that is a fused-together “platform” that is unmatched in any medical treatment.
Genetically modified T cells, or CAR T cells, are derived from a patient’s own T cells that attack cancer. To create them, several million T cells are extracted from a patient’s blood and genetically modified to generate a unique structure on their surface known as a chimeric antigen receptor, or CAR. The CAR is made to adhere to a particular antigen, or marker, on the tumor cells of a patient. The cells—which are now known as CAR T cells—are expanded in a laboratory until they reach hundreds of millions of cells. Upon reinfusion of the cells into the patient, their uniquely shaped receptor binds to the antigen on tumor cells and launches an attack on the cancer by the immune system.
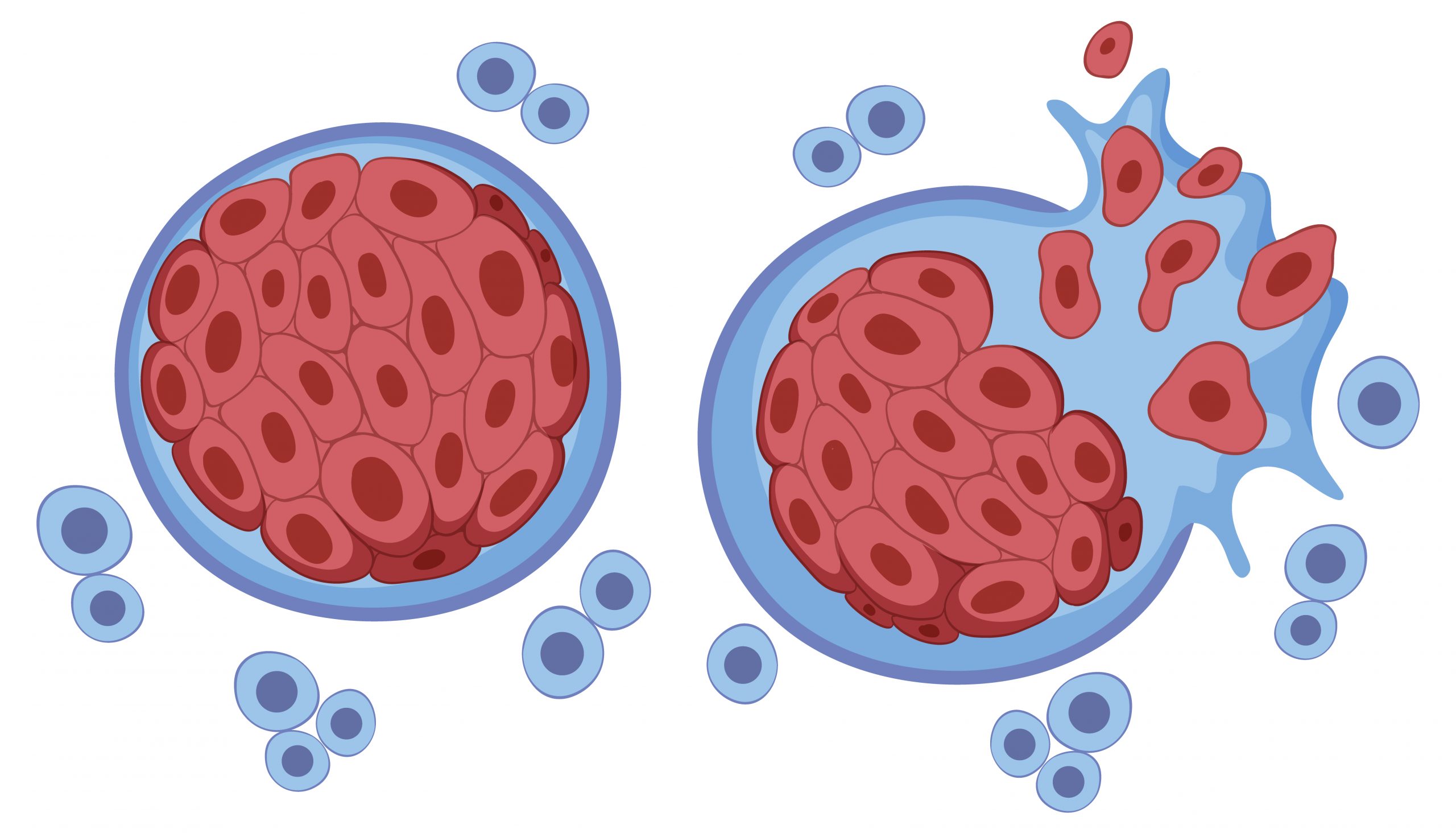
“The attack destroys nearly all the tumor cells, but a tiny percentage remains,” Rashidian explains. “The CAR T cells are effector cells: they live to kill cancer cells. When they can’t find any more to kill, they act as if their job is done and go away. The remaining tumor cells, however, can set the stage for a resurgence of the cancer.”
The CAR-E platform is a completely new therapeutic substance that the Dana-Farber researchers developed in order to extend the attack of the CAR T cells and give them memory. It is composed of the same antigen that the CAR is intended to attach to combined with a weaker version of the immune-signaling molecule interleukin-2 (IL-2).
“IL-2 has a strong effect on T cells – activating them and causing them to proliferate – but it can also be highly toxic to patients,” Rashidian remarks. “For that reason, we used a very weak form of it. On its own, it has no effect on normal T cells but has a stimulatory effect on CAR T cells when targeted specifically to them.”
The process of fusing IL-2 with a particular antigen allows for this precise targeting. The CAR attaches to an antigen on myeloma cells known as B-cell maturation antigen (BCMA) in CAR T-cell treatments for multiple myeloma. In the novel treatment, that antigen is attached to IL-2.
“Just like weak IL-2, the BCMA antigen by itself doesn’t affect CAR T cells, but, together, they have a synergy whose impact was well beyond our expectations,” says the study’s first author Taha Rakhshandehroo, PhD, of Dana-Farber.
Researchers discovered that CAR-E therapy not only increases the number of CAR T cells but also causes them to diversify, producing many CAR T cell types with various characteristics. “It generated not only effector T cells, which most patients already have, but also stem cell-like memory T cells, central memory T cells, effector memory T cells – a complete repertoire of the kinds of T cells needed for an effective immune response to cancer,” Rashidian says.
Researchers discovered that CAR-E therapy completely eliminated tumor cells, eliminating all evidence of the cancer, in myeloma cell cultures grown in the lab and in animal models of the illness.
There were further advantages. Researchers found that re-administering CAR-E could re-stimulate the long-lasting CAR T cells produced by the therapy. This implies that higher dosages of CAR-E therapy may be an effective way to treat individuals who relapse following CAR T-cell therapy. Additionally, CAR-E increases the likelihood that patients may receive treatment with fewer CAR T cells than they now do. Patients must wait several weeks before receiving an infusion of CAR T cells due to the time-consuming, costly, and resource-intensive procedure of allowing the cells to proliferate into the hundreds of millions.
One of the most frequent adverse effects of CAR T-cell therapy, cytokine release syndrome, which is a too aggressive immune response resulting in fever, nausea, fast heartbeat, neurological disorders, or other difficulties, is partially caused by the high concentrations. The process of expanding CAR T cells may not even need to occur with CAR-E; instead, CAR T cells would just need to be created and pumped into patients, who would then get CAR-E therapy.
“In animal studies, we infused mice with very low numbers of CAR T cells and found that weren’t able to clear the cancer,” Rashidian says. “When we gave them the CAR-E treatment, the CAR T cells expanded and were able to clear the cancer.”
Assurance of safety and optimization of dosage and timing of administration will be among the primary objectives of a clinical trial including CAR-E treatment. It is anticipated that the course of treatment will start about one month following the infusion of CAR T cells into the patients. A weekly dosage of CAR-E therapy would be the course of treatment for three or four weeks.
“The most exciting part of this therapy is how easily it can be integrated into the care of patients receiving CAR T-cell therapies,” says Rakhshandehroo. It’s a really classy solution to the CAR T-cell depletion issue. We can’t wait to start clinical trial testing it.”
more recommended stories
 Phage Therapy Study Reveals RNA-Based Infection Control
Phage Therapy Study Reveals RNA-Based Infection ControlKey Takeaways (Quick Summary) Researchers uncovered.
 Safer Allogeneic Stem Cell Transplants with Treg Therapy
Safer Allogeneic Stem Cell Transplants with Treg TherapyA new preclinical study from the.
 AI in Emergency Medicine and Clinician Decision Accuracy
AI in Emergency Medicine and Clinician Decision AccuracyEmergency teams rely on rapid, accurate.
 Innovative AI Boosts Epilepsy Seizure Prediction by 44%
Innovative AI Boosts Epilepsy Seizure Prediction by 44%Transforming Seizure Prediction in Epilepsy Seizure.
 Hypnosis Boosts NIV Tolerance in Respiratory Failure
Hypnosis Boosts NIV Tolerance in Respiratory FailureA New Approach: Hypnosis Improves NIV.
 Bee-Sting Microneedle Patch for Painless Drug Delivery
Bee-Sting Microneedle Patch for Painless Drug DeliveryMicroneedle Patch: A Pain-Free Alternative for.
 AI Reshapes Anticoagulation in Atrial Fibrillation Care
AI Reshapes Anticoagulation in Atrial Fibrillation CareUnderstanding the Challenge of Atrial Fibrillation.
 Hemoglobin as Brain Antioxidant in Neurodegenerative Disease
Hemoglobin as Brain Antioxidant in Neurodegenerative DiseaseUncovering the Brain’s Own Defense Against.
 Global Data Resource for Progressive MS Research (Multiple Sclerosis)
Global Data Resource for Progressive MS Research (Multiple Sclerosis)The International Progressive MS Alliance has.
 AI Diabetes Risk Detection: Early T2D Prediction
AI Diabetes Risk Detection: Early T2D PredictionA new frontier in early diabetes.

Leave a Comment