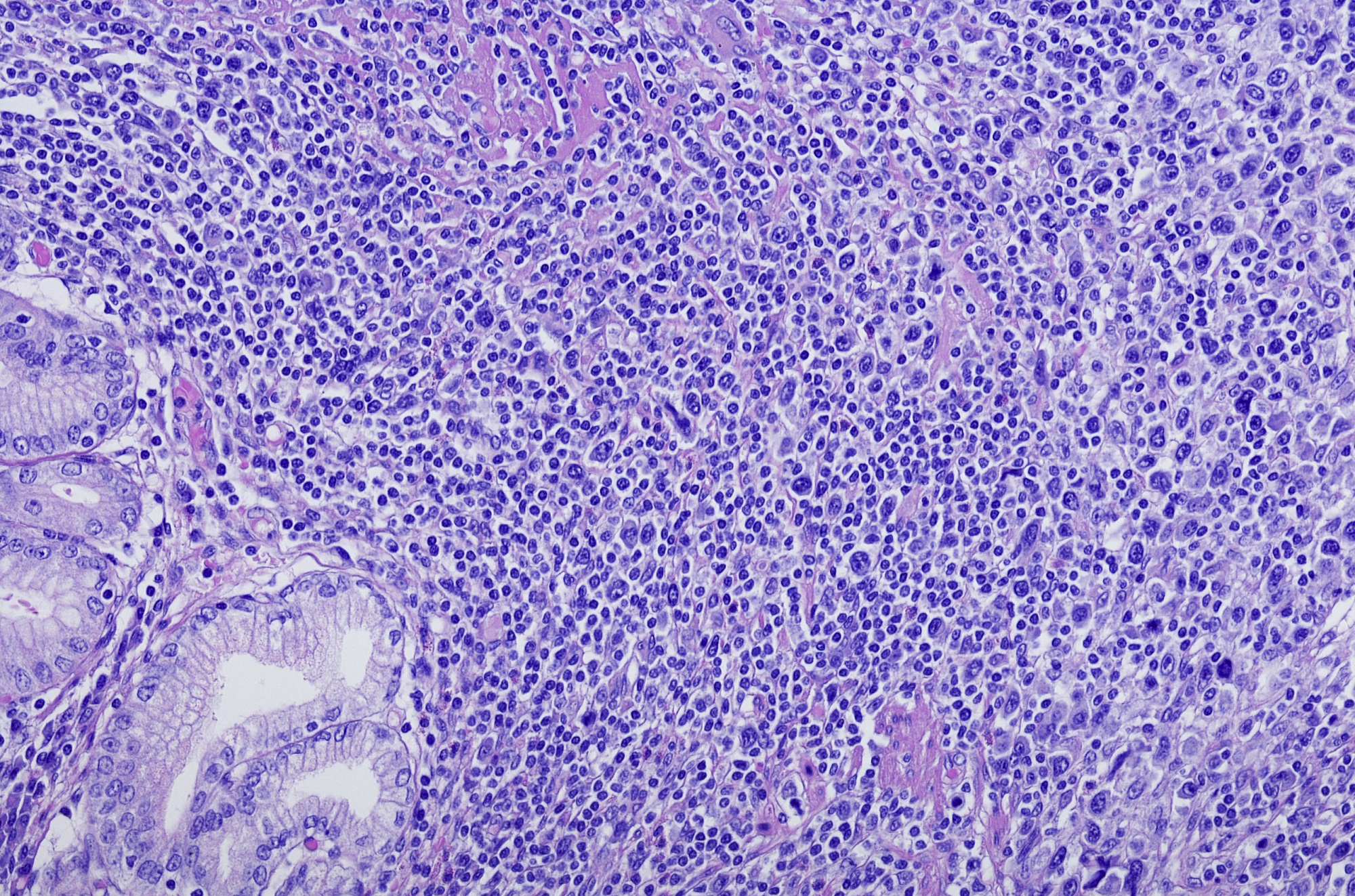

Axicabtagene ciloleucel (axi-cel) appears to be both safe and effective for treating relapsed/refractory follicular lymphoma (FL), according to research presented at the European Hematology Association (EHA) 2021 Virtual Congress.
The phase 2 ZUMA-5 trial (ClinicalTrials.gov Identifier: NCT03105336) previously demonstrated that axi-cel, a chimeric antigen receptor (CAR)-T cell therapy targeting CD19, showed a both high overall response rate and long duration of response in patients with relapsed/refractory FL, including in patients with high-risk disease.
In this presentation, the authors reported comparative clinical outcomes from updated ZUMA-5 data and the international SCHOLAR-5 cohort.
Data from the SCHOLAR-5 cohort were taken from 7 institutions, and included data from a previous trial of idelalisib; patients in SCHOLAR-5 had received a third-line or later therapy after July 2014. Patient characteristics were balanced between the ZUMA-5 and SCHOLAR-5 cohorts.
Overall, data from 86 patients in ZUMA-5 and 85 patients in SCHOLAR-5 were included; the median follow-up times were 23.3 months and 26.2 months, respectively. Patients in the SCHOLAR-5 cohort had more patients with an Eastern Cooperative Oncology Group score of 1 at baseline, despite attempts to match characteristics.
In patients who had received at least 2 prior therapy lines, the overall response rates were 49.9% in SCHOLAR-5 vs 94.2% in ZUMA-5 (odds ratio, 16.2; 95% CI, 5.6-46.9). The median progression-free survival and overall survival periods were not reached in ZUMA-5, and were 12.7 months and 59.8 months, respectively, in SCHOLAR-5; the corresponding hazard ratios for progression-free and overall survival were 0.30 (95% CI, 0.18-0.49) and 0.42 (95% CI, 0.21-0.83), respectively.
“These data support that axi-cel represents a significant improvement in treatment options for patients with relapsed/refractory FL,” the presenter said.
Disclosure: The presenter declared affiliations with AstraZeneca, Kite, A Gilead Company, Gilead, Abbvie, Bristol Myers Squibb, MorphoSys, Novartis, Takeda, TG Therapeutics, Janssen.
Reference
Ghione P, Anik Patel A, Bobillo S, et al. A comparison of clinical outcomes from ZUMA-5 (axicabtagene ciloleucel) and the international SCHOLAR-5 external control cohort in relapsed/refractory follicular lymphoma (R/R FL). Paper presented at: European Hematology Association 2021 Virtual Congress; June 2021; Abstract LB1904.
The post Relapsed, Refractory Follicular Lymphoma: CAR-T Cell Therapy Shows Promise appeared first on Cancer Therapy Advisor.
more recommended stories
 Pancreatic Cancer Research: Triple-Drug Therapy Success
Pancreatic Cancer Research: Triple-Drug Therapy SuccessKey Summary Spanish researchers report complete.
 Immune Cell Epigenome Links Genetics and Life Experience
Immune Cell Epigenome Links Genetics and Life ExperienceKey Takeaway Summary Immune cell responses.
 Dietary Melatonin Linked to Depression Risk: New Study
Dietary Melatonin Linked to Depression Risk: New StudyKey Summary Cross-sectional analysis of 8,320.
 Chronic Pain Linked to CGIC Brain Circuit, Study Finds
Chronic Pain Linked to CGIC Brain Circuit, Study FindsKey Takeaways University of Colorado Boulder.
 New Insights Into Immune-Driven Heart Failure Progression
New Insights Into Immune-Driven Heart Failure ProgressionKey Highlights (Quick Summary) Progressive Heart.
 Microplastic Exposure and Parkinson’s Disease Risk
Microplastic Exposure and Parkinson’s Disease RiskKey Takeaways Microplastics and nanoplastics (MPs/NPs).
 Sickle Cell Gene Therapy Access Expands Globally
Sickle Cell Gene Therapy Access Expands GloballyKey Summary Caring Cross and Boston.
 Reducing Alcohol Consumption Could Lower Cancer Deaths
Reducing Alcohol Consumption Could Lower Cancer DeathsKey Takeaways (At a Glance) Long-term.
 NeuroBridge AI Tool for Autism Communication Training
NeuroBridge AI Tool for Autism Communication TrainingKey Takeaways Tufts researchers developed NeuroBridge,.
 Population Genomic Screening for Early Disease Risk
Population Genomic Screening for Early Disease RiskKey Takeaways at a Glance Population.

Leave a Comment