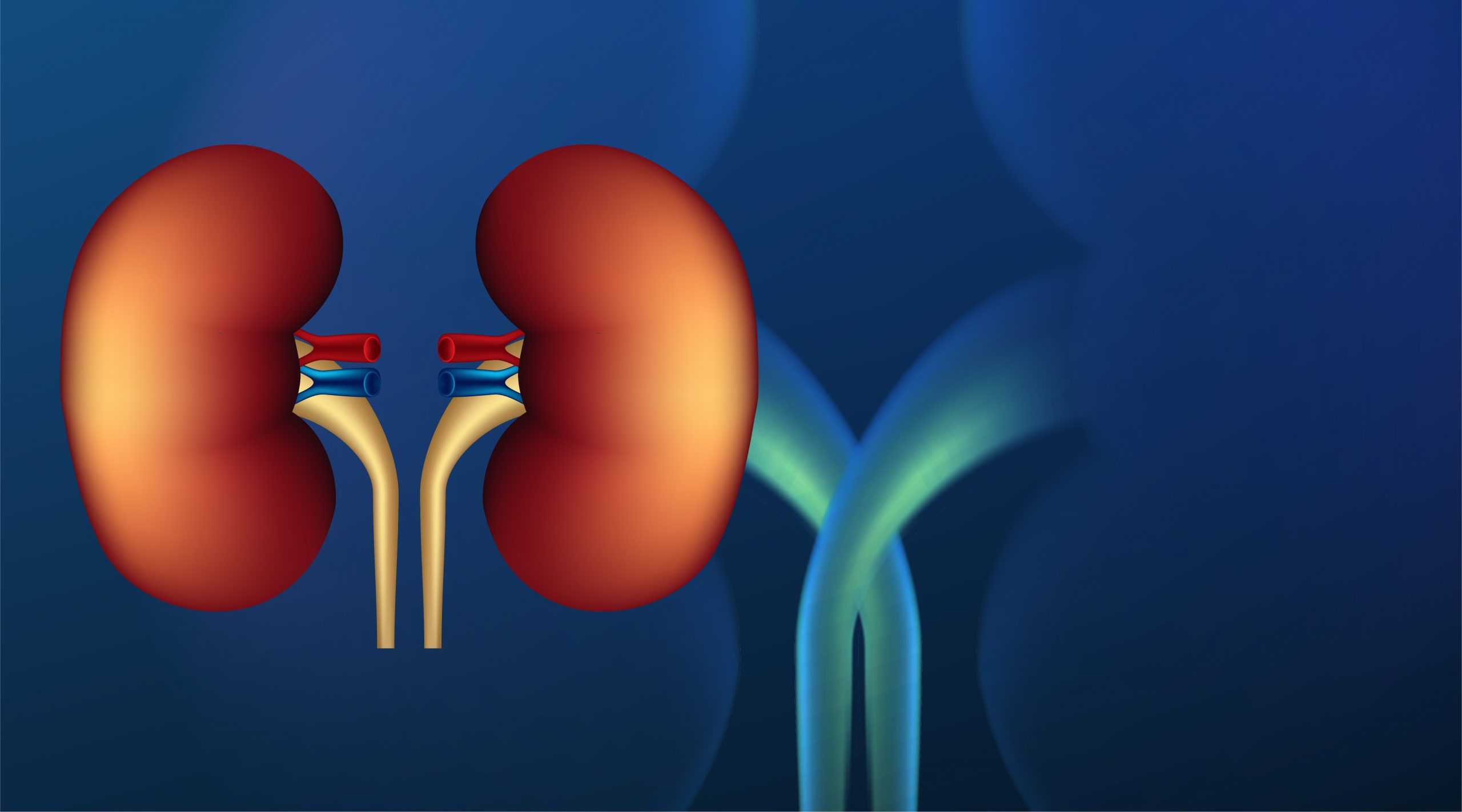

Researchers at the University of Minnesota have successfully transplanted frozen rat kidneys after allowing them to defrost from a 100-day freeze in a pioneering new study. This kidney transplant is incredibly significant for the medical field since it could demonstrate the life-saving feasibility of long-term, low-temperature organ preservation.
This breakthrough has the potential to save thousands of lives. The paper, published in Nature Communications, describes past challenges posed by the production of ice crystals that harm live tissue when frozen, as well as the irreversible damage caused by uneven thawing. Five transplants in rats were successful, with the kidneys working within 30 days after being frozen.
“This is the first time anyone has published a robust protocol for long-term storage, rewarming and successful transplantation of a functional preserved organ in an animal,” says Dr. John Bischof, a mechanical engineering professor and director of the U of M Institute of Engineering in Medicine. This study has been in the works for over a decade, and effective cryopreservation has been a goal in many medical, biological and agricultural industries for quite some time. But when it comes to organ preservation, this could be huge.
About 20% of donor kidneys go to waste because they are not delivered to recipients in time. Cryopreservation has been practiced for decades, but the major challenge has been rewarming the organs without causing significant ice damage or cell rupture.
The researchers created a specific nano-warming technology that uses tiny particles called iron oxide nanoparticles to warm organs swiftly and evenly. These nanoparticles are suspended in a specific liquid that shields the organ as it warms up. The nanoparticle-containing liquid is pushed through the organ’s blood arteries. The nanoparticles are then activated using specific waves, which heats up the organ. This procedure permits the organ to be warmed from within rather than only from the outside.
In this study, postdoctoral researchers Zonghu Han (mechanical engineering) and Joseph Sushil Rao (surgery) demonstrated that rat kidneys can be cryogenically stored for up to 100 days, successfully rewarmed, cleared of cryoprotective fluids and nanoparticles, and transplanted into rats. When the kidneys were transplanted, the five rat recipients were able to regain full renal function within 30 days without any extra procedures.
“During the first two to three weeks, the kidneys weren’t at full function, but by three weeks, they recovered. By one month, they were fully functioning kidneys that were completely indistinguishable from transplants of a fresh organ,” said the study’s co-senior author Erik Finger, a transplant surgeon and professor of surgery at the University of Minnesota Medical School.
Finger stated that with human transplants, considerably more interventions would take place, such as drugs and dialysis to support the kidney in the weeks following transplantation. None of this was done to the rats, and they still survived the procedure with full kidney function restored.
“We have been working on this process for years to make sure everything was in place before we transplanted into a rat,” said Michael Etheridge, a principal research engineer in the University’s department of mechanical engineering. “Still, it is a very complicated process. We weren’t surprised this worked, but we weren’t going to be surprised if it didn’t work. I’m very proud of our team.”
Long-term organ banking could improve donor organ use, donor/recipient matching, immunological tolerance protocols (cutting the need for immunosuppressive medicines), and surgery preparation and scheduling. Importantly, while this method has been established in the kidney, it may one day be applied to other transplant organs and allow for long-term storage of organs and tissue for biological and pharmacological research.
The researchers have demonstrated that all components of this approach can be scaled to larger organs, and they plan to demonstrate the process using pig kidneys next. While it will be some years before a cryopreserved organ is transplanted into people, the team is optimistic that it will be possible in the future.
The research was primarily supported by the National Institutes of Health, with additional funding provided by the National Science Foundation and the Biostasis Research Institute, which was financed in part by LifeGift, Nevada Donor Network, Lifesource, Donor Network West, and Lifebanc.
more recommended stories
 Chronic Pain Linked to CGIC Brain Circuit, Study Finds
Chronic Pain Linked to CGIC Brain Circuit, Study FindsKey Takeaways University of Colorado Boulder.
 New Insights Into Immune-Driven Heart Failure Progression
New Insights Into Immune-Driven Heart Failure ProgressionKey Highlights (Quick Summary) Progressive Heart.
 Microplastic Exposure and Parkinson’s Disease Risk
Microplastic Exposure and Parkinson’s Disease RiskKey Takeaways Microplastics and nanoplastics (MPs/NPs).
 Sickle Cell Gene Therapy Access Expands Globally
Sickle Cell Gene Therapy Access Expands GloballyKey Summary Caring Cross and Boston.
 Reducing Alcohol Consumption Could Lower Cancer Deaths
Reducing Alcohol Consumption Could Lower Cancer DeathsKey Takeaways (At a Glance) Long-term.
 NeuroBridge AI Tool for Autism Communication Training
NeuroBridge AI Tool for Autism Communication TrainingKey Takeaways Tufts researchers developed NeuroBridge,.
 Population Genomic Screening for Early Disease Risk
Population Genomic Screening for Early Disease RiskKey Takeaways at a Glance Population.
 Type 2 Diabetes Risk Identified by Blood Metabolites
Type 2 Diabetes Risk Identified by Blood MetabolitesKey Takeaways (Quick Summary) Researchers identified.
 Microglia Neuroinflammation in Binge Drinking
Microglia Neuroinflammation in Binge DrinkingKey Takeaways (Quick Summary for HCPs).
 Precision Oncology with Personalized Cancer Drug Therapy
Precision Oncology with Personalized Cancer Drug TherapyKey Takeaways UC San Diego’s I-PREDICT.

Leave a Comment