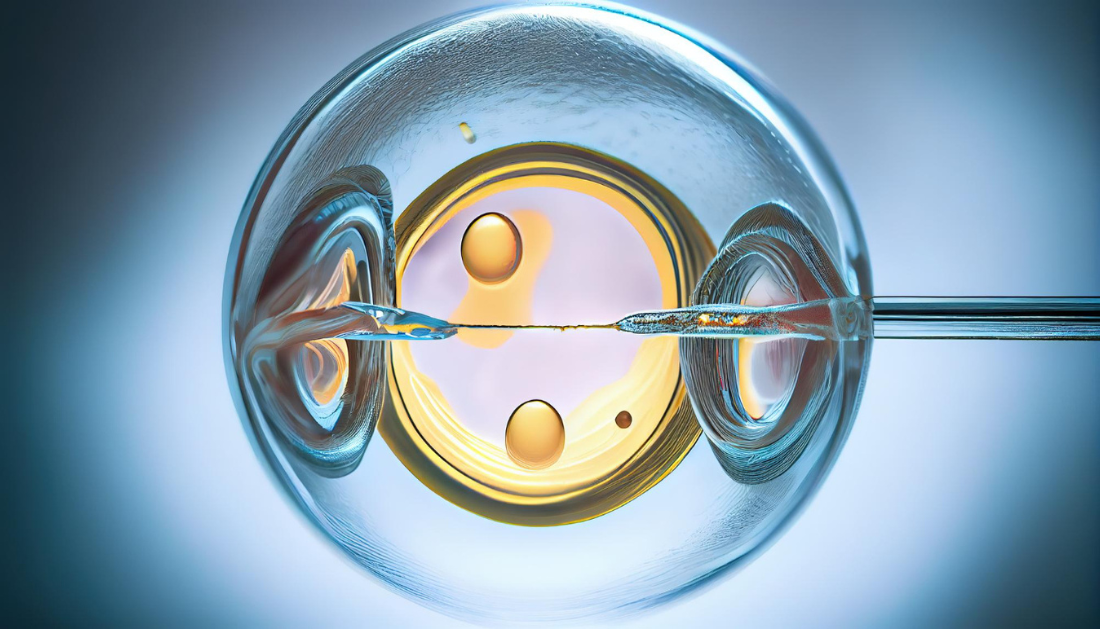

Infertility affects around 48 million couples globally and can be caused by a variety of factors. The ovary produces eggs in mammals, including humans. When this mechanism fails, it can result in female infertility. Premature ovarian insufficiency, which causes issues with egg production before the age of 40, is one example of this. Infertility affects up to 3.7% of females, with genetic abnormalities accounting for approximately 30% of instances.
Professor Kehkooi Kee of Tsinghua University in China, who collaborated on a recent study on this subject, has been researching this illness for some years.
“In 2019, our collaborators, Professor Li’s team, encountered a family with premature ovarian insufficiency in which changes to a gene called Eif4enif1 appeared to be responsible for the disease,” Dr. Kee said.
The researchers decided to replicate this genetic alteration in mice in order to learn more about how it affects human fertility. They demonstrate that modifications to these mice’s mitochondria—the cell’s powerhouses—have an effect on their eggs. Their research on this novel discovery has been published in Development.
CRISPR was utilized by the researchers to introduce the genetic alteration into the mice. They allowed these mice to mature before comparing their fertility to that of mice whose DNA had not been altered. The study’s first author, Yuxi Ding, an M.D./Ph.D. student, discovered that the average number of total follicles (the tiny sacs that house maturing eggs) was reduced by around 40% in older and genetically modified mice. The average number of pups in each litter was reduced by 33%.
Importantly, when cultured in a plate, almost half of the fertilized eggs did not survive past the early phases of development. This indicated that, like the human patients, these mice were having fertility issues.
When the researchers examined the mice eggs under the microscope, they discovered something peculiar about their mitochondria. Mitochondria generate the energy that cells, particularly egg cells, require. Mitochondria are normally dispersed widely throughout the egg, however mitochondria in mouse eggs with the genetic mutation were crowded together.
“We were actually surprised by the differences in mitochondria,” Professor Kee stated. “At the time we were doing this research, a link between Eif4enif1 and mitochondria had not been seen before.”
These misbehaving mitochondria appear to be contributing to the fertility issues in these mice, prompting the researchers to postulate that restoring appropriate mitochondrial behavior may increase fertility.
This study points the way forward for future research in human infertility, such as determining whether mitochondrial defects are found in the eggs of human patients with premature ovarian insufficiency, as well as whether these same mitochondrial defects are observed in embryos after the eggs are fertilized. Furthermore, determining if restoring normal mitochondrial distribution increases fertility could lead to a novel therapeutic method.
“Our research suggests that rescuing oocyte mitochondria abnormality could be a potential therapeutic target for clinical infertility patients with genetic variants,” Prof. Kee said.
more recommended stories
 Brain Pulsations Linked to High BMI
Brain Pulsations Linked to High BMIAccording to a new study from.
 Brain Age Estimation: EEG Advancements in Neurology
Brain Age Estimation: EEG Advancements in NeurologyTo estimate brain age using EEG.
 Unlocking Ketogenic Diet for Epilepsy Management
Unlocking Ketogenic Diet for Epilepsy ManagementExploring the Therapeutic Potential of Ketogenic.
 Senescence in Neurons: Findings
Senescence in Neurons: FindingsBased on a new study by.
 Balanced Diet Linked to Enhanced Brain Health
Balanced Diet Linked to Enhanced Brain HealthDiet and brain health are strongly.
 Acid-Reducing Drugs Linked to Higher Migraine Risk
Acid-Reducing Drugs Linked to Higher Migraine RiskIndividuals who utilize acid-reducing drugs may.
 Atrial Fibrillation in Young Adults: Increased Heart Failure and Stroke Risk
Atrial Fibrillation in Young Adults: Increased Heart Failure and Stroke RiskIn a recent study published in.
 Neurodegeneration Linked to Fibrin in Brain Injury
Neurodegeneration Linked to Fibrin in Brain InjuryThe health results for the approximately.
 DELiVR: Advancing Brain Cell Mapping with AI and VR
DELiVR: Advancing Brain Cell Mapping with AI and VRDELiVR is a novel AI-based method.
 Retinal Neurodegeneration in Parkinson’s Disease
Retinal Neurodegeneration in Parkinson’s DiseaseBy measuring the thickness of the.

Leave a Comment