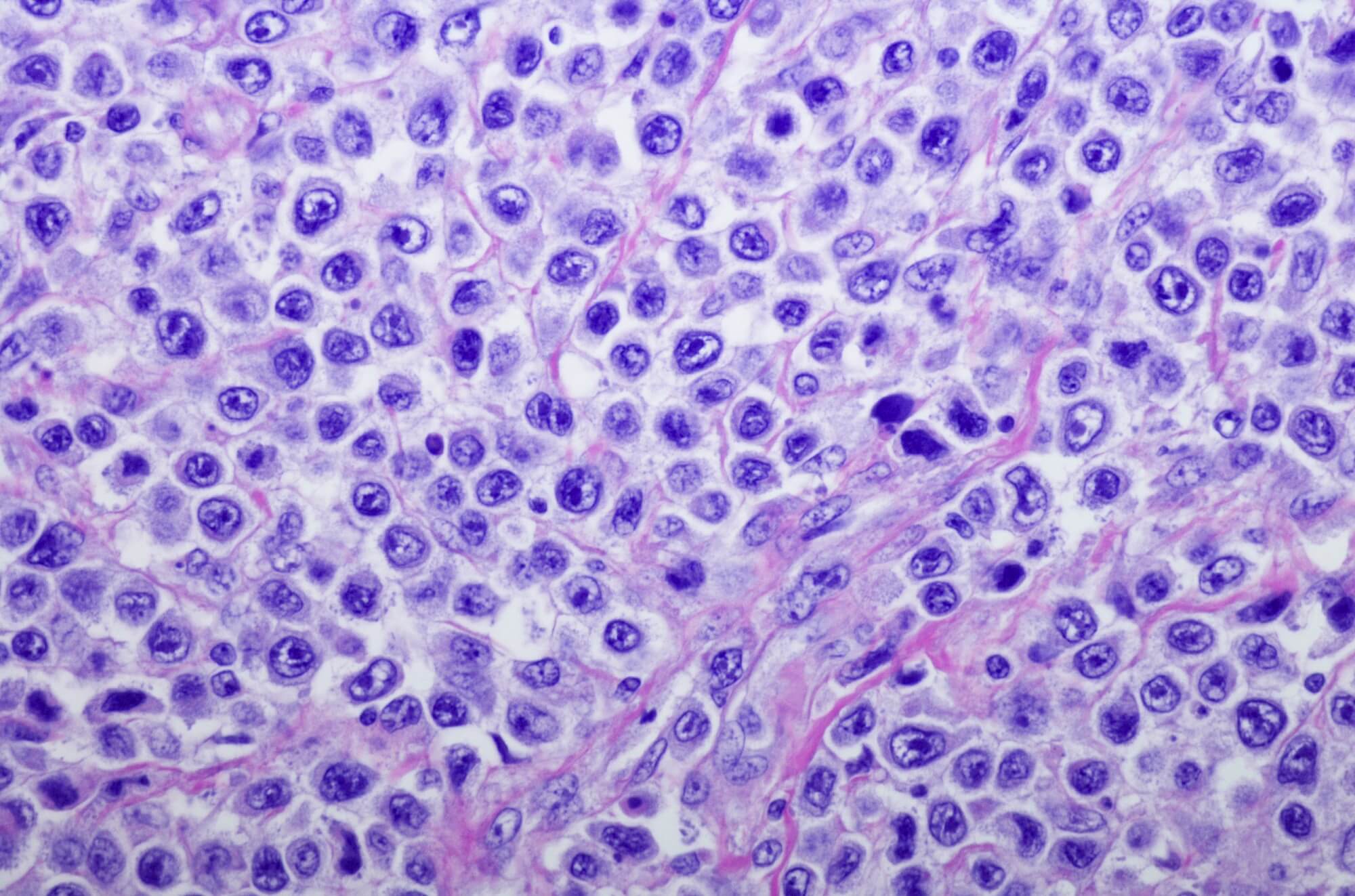

Among patients with diffuse large B cell lymphoma (DLBCL) and other non-Hodgkin B cell lymphomas (B-NHLs), naratuximab emtansine appears to be a both safe and effective treatment, according to research presented at the European Hematology Association (EHA) 2021 Virtual Congress.
Naratuximab (Debio 1562), a CD37-targeting antibody, showed activity in B-NHL in preclinical models when combined with rituximab. CD37 is a lymphocyte surface marker that is highly expressed on DLBCL and other B-NHLs. Targeting CD37 is therefore a promising therapeutic option in the B-NHL disease setting, and previous phase 1 study suggested naratuximab may be both efficacious and safe in DLBCL.
For the present phase 2 study (ClinicalTrials.gov Identifier: NCT02564744), researchers evaluated the safety and efficacy of naratuximab with rituximab among patients with relapsed/refractory DLBCL and other B-NHL subtypes. Patients were assigned to either cohort A (50 patients), which involved treatment once every 3 weeks, or to cohort B (30 patients), which was a weekly regimen. All included patients were transplant-ineligible and had received 1 to 6 prior lines of therapy.
Overall, 100 patients (80 DLBCL, 20 other B-NHL subtypes) were enrolled and received naratuximab with rituximab. Of the 80 patients with DLBCL, 10 (12.5%) had primary refractory disease, 24 (30%) were refractory to their last therapy line, and 35 (44%) had received at least 2 prior systemic therapies.
Seventy-six of the 80 patients with DLBCL were evaluable for efficacy. The overall response rate was 44.7% after a median follow-up of 15 months, and 24 (31.6%) patients had a complete response. Thirty-three (43.4%) patients had progressive disease. Naratuximab treatment was, furthermore, linked with full peripheral target engagement and B cell depletion.
The overall response rate was 50% in cohorts A and B, though more patients in cohort A (43.3%) had a complete response than in cohort B (33.3%).
Of the overall population, 81% of patients had a grade 3 or worse treatment-related adverse event, including neutropenia 54 (54%), leukopenia 19 (19%), lymphopenia 17 (17%), and thrombocytopenia 12 (12%); 8 (8%) patients discontinued treatment because of an adverse event.
Disclosure: Some [or one] study author(s) declared affiliations with biotech, pharmaceutical, and/or device companies. Please see the original reference for a full list of authors’ disclosures.
Reference
Levy MY, Grudeva-Popova Z, Trneny M, et al. Safety and efficacy of CD37-targeting naratuximab emtansine plus rituximab in diffuse large B-cell lymphoma and other non-Hodgkin’s B-cell lymphomas – a phase 2 study. Paper presented at: European Hematology Association 2021 Virtual Congress. Abstract LB1903.
The post DLBCL: Naratuximab Shows Promise Among Patients With Relapsed/Refractory Disease appeared first on Cancer Therapy Advisor.
more recommended stories
 Pancreatic Cancer Research: Triple-Drug Therapy Success
Pancreatic Cancer Research: Triple-Drug Therapy SuccessKey Summary Spanish researchers report complete.
 Immune Cell Epigenome Links Genetics and Life Experience
Immune Cell Epigenome Links Genetics and Life ExperienceKey Takeaway Summary Immune cell responses.
 Dietary Melatonin Linked to Depression Risk: New Study
Dietary Melatonin Linked to Depression Risk: New StudyKey Summary Cross-sectional analysis of 8,320.
 Chronic Pain Linked to CGIC Brain Circuit, Study Finds
Chronic Pain Linked to CGIC Brain Circuit, Study FindsKey Takeaways University of Colorado Boulder.
 New Insights Into Immune-Driven Heart Failure Progression
New Insights Into Immune-Driven Heart Failure ProgressionKey Highlights (Quick Summary) Progressive Heart.
 Microplastic Exposure and Parkinson’s Disease Risk
Microplastic Exposure and Parkinson’s Disease RiskKey Takeaways Microplastics and nanoplastics (MPs/NPs).
 Sickle Cell Gene Therapy Access Expands Globally
Sickle Cell Gene Therapy Access Expands GloballyKey Summary Caring Cross and Boston.
 Reducing Alcohol Consumption Could Lower Cancer Deaths
Reducing Alcohol Consumption Could Lower Cancer DeathsKey Takeaways (At a Glance) Long-term.
 NeuroBridge AI Tool for Autism Communication Training
NeuroBridge AI Tool for Autism Communication TrainingKey Takeaways Tufts researchers developed NeuroBridge,.
 Population Genomic Screening for Early Disease Risk
Population Genomic Screening for Early Disease RiskKey Takeaways at a Glance Population.

Leave a Comment