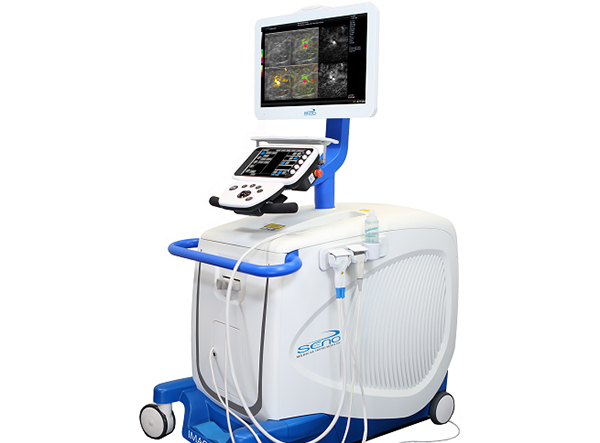

Seno Medical, a San Antonio, Texas firm, has won FDA approval to introduce its Imagio device that utilizes optoacoustic imaging to help physicians identify whether breast lesions are benign or malignant. It is hoped that this non-invasive and radiation-free technology will help to reduce the number of biopsies that have to be performed.

Opto-acoustic imaging combines ultrasound with laser optics to provide a combined view of tissue anatomy and the blood flow around it. Cancerous lesions commonly exhibit a high density of newly formed blood vessels (angiogenesis) and a lower oxygen saturation compared with surrounding tissues. The Imagio system offers a look at these parameters in real-time and radiologists, trained and certified to have a keen eye and with the help of accompanying AI software called SenoGram, should be able to differentiate between malignant and benign breast lesions in many cases.
At RSNA back in 2017 in Chicago, we interviewed Dr. Tom Stavros, Seno’s Chief Medical Officer, who introduced us to the Imagio system and showed off its imaging capabilities. The device was already cleared for use in Europe back then. Here’s what Dr. Stavros said in a statement following this FDA approval, in a press release: “Optimizing the diagnosis of breast masses requires a combination of very high sensitivity (≥98%) while simultaneously maximizing specificity and minimizing false positives and biopsies of benign masses. Other modalities have reported improvements in specificity, but these have often come at the expense of the desired high ≥98% sensitivity. The data from the PMA study shows that OA/US successfully achieved improved specificity at a fixed sensitivity of 98%, the part of the ROC curve where clinical decisions about whether or not to biopsy a mass is actually made.”
more recommended stories
 Texas Medical Board Releases Abortion Training for Physicians
Texas Medical Board Releases Abortion Training for PhysiciansKey Takeaways Texas Medical Board has.
 Safer Allogeneic Stem Cell Transplants with Treg Therapy
Safer Allogeneic Stem Cell Transplants with Treg TherapyA new preclinical study from the.
 Autoimmune Disorders: ADA2 as a Therapeutic Target
Autoimmune Disorders: ADA2 as a Therapeutic TargetAdenosine deaminase 2 (ADA2) has emerged.
 Kaempferol: A Breakthrough in Allergy Management
Kaempferol: A Breakthrough in Allergy ManagementKaempferol, a dietary flavonoid found in.
 Early Milk Cereal Drinks May Spur Infant Weight Gain
Early Milk Cereal Drinks May Spur Infant Weight GainNew research published in Acta Paediatrica.
 TaVNS: A Breakthrough for Chronic Insomnia Treatment
TaVNS: A Breakthrough for Chronic Insomnia TreatmentA recent study conducted by the.
 First-of-Its-Kind Gene-Edited Pig Kidney: Towana’s New Life
First-of-Its-Kind Gene-Edited Pig Kidney: Towana’s New LifeSurgeons at NYU Langone Health have.
 Just-in-Time Training Improves Success & Patient Safety
Just-in-Time Training Improves Success & Patient SafetyA study published in The BMJ.
 ChatGPT Excels in Medical Summaries, Lacks Field-Specific Relevance
ChatGPT Excels in Medical Summaries, Lacks Field-Specific RelevanceIn a recent study published in.
 Study finds automated decision minimizes high-risk medicine combinations in ICU patients
Study finds automated decision minimizes high-risk medicine combinations in ICU patientsA multicenter study coordinated by Amsterdam.

Leave a Comment