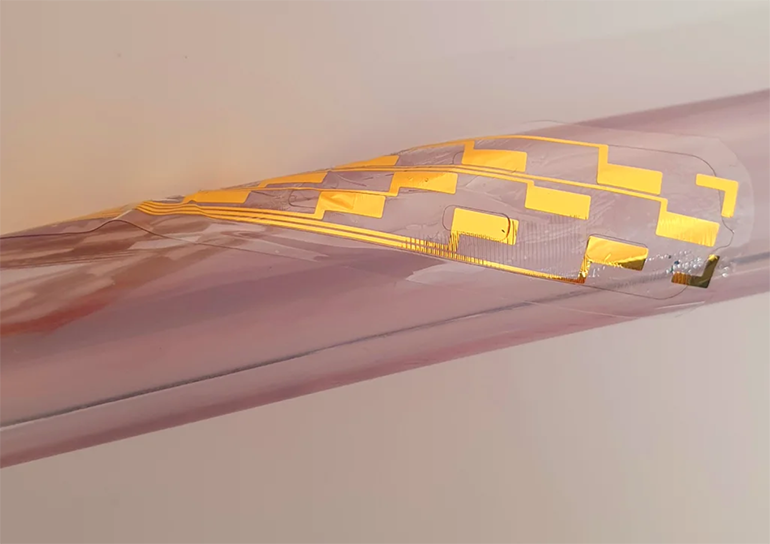

Researchers at the University of Cambridge created a spinal stimulation device that can help to control severe pain. Unlike existing technologies, which require invasive surgery for implantation, the new device can be delivered using a needle. Once implanted, the device unfurls and inflates to provide extensive coverage during spinal cord stimulation to control severe pain. The technology could make spinal cord stimulation more accessible for millions of chronic pain sufferers.
For many patients, chronic pain does not respond to conventional treatments and can be severe and debilitating. For such individuals, spinal cord stimulation may be an option, whereby electricity is used to disrupt pain signals in the spine. However, installing the most effective type of spinal stimulation devices is invasive, requiring complex surgery under general anesthesia, and many patients are poor candidates for such treatment.
“Spinal cord stimulation is a treatment of last resort, for those whose pain has become so severe that it prevents them from carrying out everyday activities,” said Damiano Barone, a researcher involved in the study, via a University of Cambridge announcement. “However, the two main types of spinal cord stimulation devices both have flaws, which may be one reason their use is limited, even though millions struggle with chronic pain every day.”
This latest technology aims to combine minimally invasive delivery with extensive spinal coverage and efficacy in pain relief. “Our goal was to make something that’s the best of both worlds – a device that’s clinically effective, but that doesn’t require complex and risky surgery,” said Christopher Proctor, another researcher involved in the study. “This could help bring this life-changing treatment option to many more people.”

The device is an ultrathin pouch rolled into a tiny cylinder that can fit into a needle. Once delivered into the epidural space, the pouch unrolls and can be inflated using water or air to cover an area of the spinal cord. Tiny electrodes within the inflated structure can then deliver electrical current to the spinal cord, helping to disrupt pain signals.
“The way we make the device means that we can also incorporate additional components – we could add more electrodes or make it bigger to cover larger areas of the spine with increased accuracy,” said Barone. “This adaptability could make our spinal cord stimulation device a potential treatment for paralysis following spinal cord injury, stroke or movement disorders such as Parkinson’s disease. In addition, an effective device that doesn’t require invasive surgery could bring relief to so many people.”
Here’s a video demonstrating the new device expanding:
more recommended stories
 Pancreatic Cancer Research: Triple-Drug Therapy Success
Pancreatic Cancer Research: Triple-Drug Therapy SuccessKey Summary Spanish researchers report complete.
 Immune Cell Epigenome Links Genetics and Life Experience
Immune Cell Epigenome Links Genetics and Life ExperienceKey Takeaway Summary Immune cell responses.
 Dietary Melatonin Linked to Depression Risk: New Study
Dietary Melatonin Linked to Depression Risk: New StudyKey Summary Cross-sectional analysis of 8,320.
 Chronic Pain Linked to CGIC Brain Circuit, Study Finds
Chronic Pain Linked to CGIC Brain Circuit, Study FindsKey Takeaways University of Colorado Boulder.
 New Insights Into Immune-Driven Heart Failure Progression
New Insights Into Immune-Driven Heart Failure ProgressionKey Highlights (Quick Summary) Progressive Heart.
 Microplastic Exposure and Parkinson’s Disease Risk
Microplastic Exposure and Parkinson’s Disease RiskKey Takeaways Microplastics and nanoplastics (MPs/NPs).
 Sickle Cell Gene Therapy Access Expands Globally
Sickle Cell Gene Therapy Access Expands GloballyKey Summary Caring Cross and Boston.
 Reducing Alcohol Consumption Could Lower Cancer Deaths
Reducing Alcohol Consumption Could Lower Cancer DeathsKey Takeaways (At a Glance) Long-term.
 NeuroBridge AI Tool for Autism Communication Training
NeuroBridge AI Tool for Autism Communication TrainingKey Takeaways Tufts researchers developed NeuroBridge,.
 Population Genomic Screening for Early Disease Risk
Population Genomic Screening for Early Disease RiskKey Takeaways at a Glance Population.

Leave a Comment