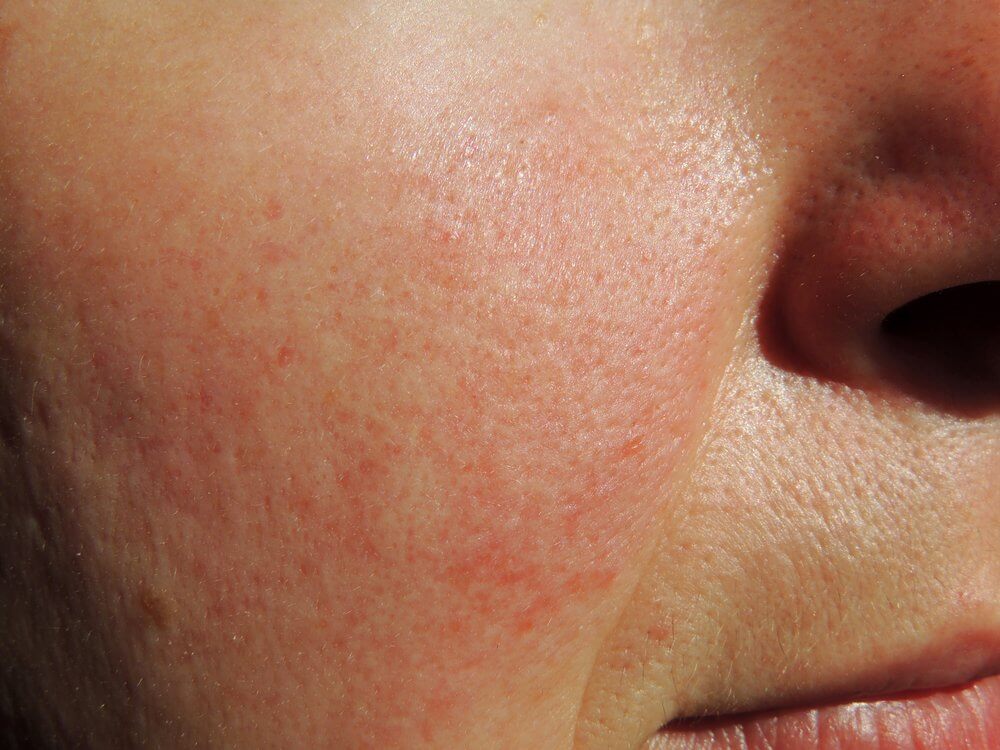

Patients experienced a progressive improvement in rosacea after treatment with a benzoyl peroxide formulation, according to results from an extension of two phase 3 trials presented at Maui Derm for Dermatologists.
“In a new microencapsulated formulation (E-BPO cream 5%), the drug is entrapped in silica microcapsules,” Neal Bhatia, MD, FAAD, and colleagues wrote in the poster. “This extends drug delivery time to improve efficacy and potently reduces the potential for skin irritation.”
Five hundred thirty-five patients (median age, 53 years; women, 71.4%) were included in the extension study; 172 patients previously received the vehicle in phase 3 trials, while 363 previously received treatment with the cream. Patients had moderate to severe rosacea and baseline Investigator’s Global Assessment score of 3 or 4, 15 to 70 inflammatory lesions, and two or fewer nodules. Outcome measures included IGA status of “clear or almost clear” at week 40, time to re-treatment, number of re-treatments and tolerability.
After 40 weeks of treatment, 67.2% of all patients (66.5% previously in vehicle group; 67.6% previously in benzoyl peroxide group) met the IGA efficacy outcome.
The median time to re-treatment was 58 days (95% CI, 57.0-64.0), with a mean number of 1.4 re-treatments.
The percentage of patients with no or mild erythema increased from 10.1% at baseline to 76.2% at 40 weeks, while the proportion of patients with no or mild telangiectasia increased from 58.2% to 80.1%.
Ninety-six patients reported a mild treatment-related adverse event, 81 reported a moderate event, and eight reported a serious event. Most were not thought to be related to the study treatment.
“The results from this long-term extension of two-phase 3 randomized controlled trials demonstrated progressive clinical improvement as reflected by the percentage of patients achieving IGA success and reduction in erythema as well as good cutaneous safety and tolerability with E-BPO cream 5% applied for up to 52 weeks in patients with rosacea,” the researchers wrote.
more recommended stories
 Texas Medical Board Releases Abortion Training for Physicians
Texas Medical Board Releases Abortion Training for PhysiciansKey Takeaways Texas Medical Board has.
 Safer Allogeneic Stem Cell Transplants with Treg Therapy
Safer Allogeneic Stem Cell Transplants with Treg TherapyA new preclinical study from the.
 Autoimmune Disorders: ADA2 as a Therapeutic Target
Autoimmune Disorders: ADA2 as a Therapeutic TargetAdenosine deaminase 2 (ADA2) has emerged.
 Kaempferol: A Breakthrough in Allergy Management
Kaempferol: A Breakthrough in Allergy ManagementKaempferol, a dietary flavonoid found in.
 Early Milk Cereal Drinks May Spur Infant Weight Gain
Early Milk Cereal Drinks May Spur Infant Weight GainNew research published in Acta Paediatrica.
 TaVNS: A Breakthrough for Chronic Insomnia Treatment
TaVNS: A Breakthrough for Chronic Insomnia TreatmentA recent study conducted by the.
 First-of-Its-Kind Gene-Edited Pig Kidney: Towana’s New Life
First-of-Its-Kind Gene-Edited Pig Kidney: Towana’s New LifeSurgeons at NYU Langone Health have.
 Just-in-Time Training Improves Success & Patient Safety
Just-in-Time Training Improves Success & Patient SafetyA study published in The BMJ.
 ChatGPT Excels in Medical Summaries, Lacks Field-Specific Relevance
ChatGPT Excels in Medical Summaries, Lacks Field-Specific RelevanceIn a recent study published in.
 Study finds automated decision minimizes high-risk medicine combinations in ICU patients
Study finds automated decision minimizes high-risk medicine combinations in ICU patientsA multicenter study coordinated by Amsterdam.

Leave a Comment