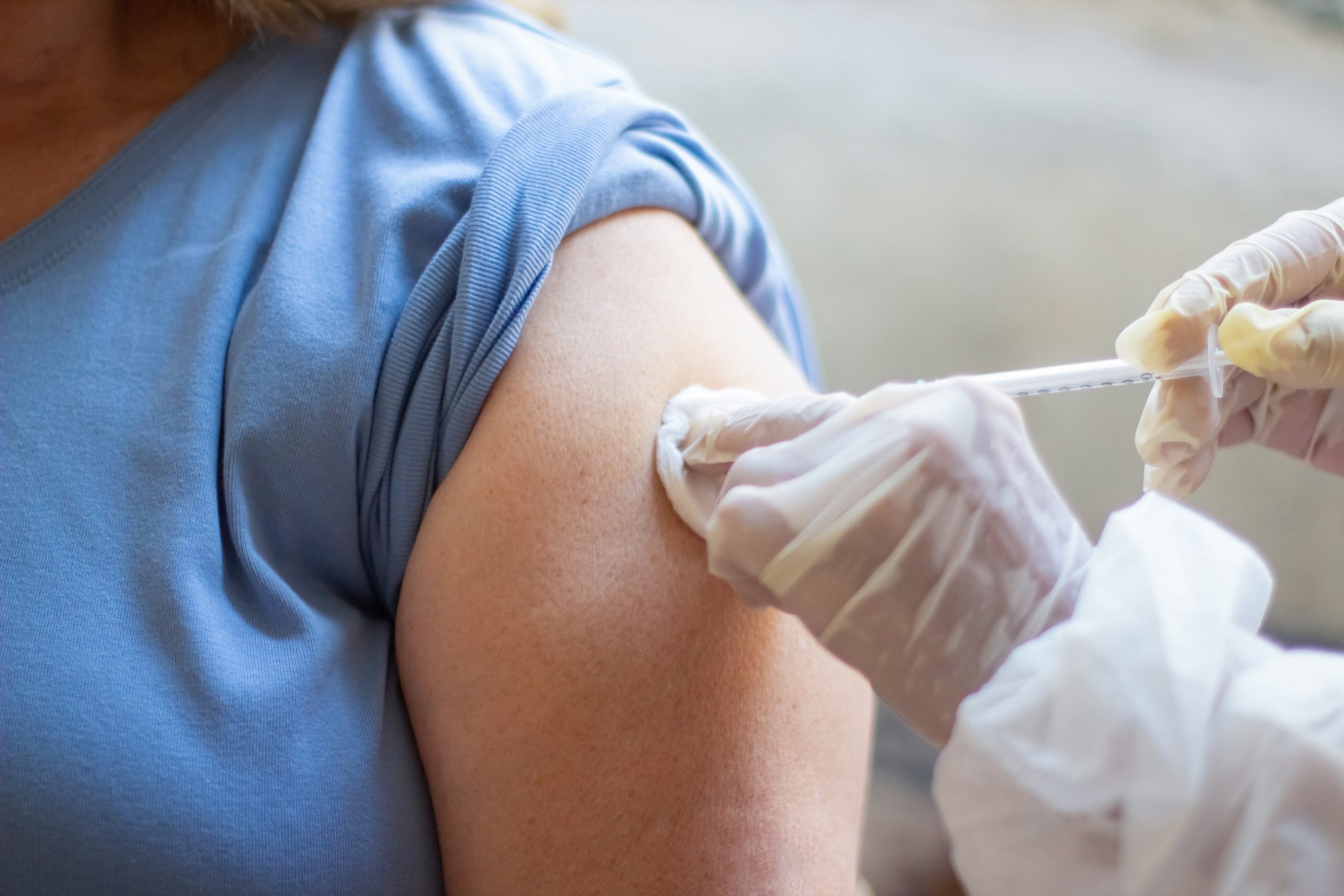

The World Health Organization’s Strategic Advisory Group of Experts (SAGE) and the Malaria Policy Advisory Group (MPAG) have both advised using the R21/Matrix-MTM malaria vaccine.
R21/Matrix-MTM malaria vaccine, developed by The University of Oxford and the Serum Institute of India using Novavax’s adjuvant technology, exhibits great efficacy with a reassuring safety profile.
The R21/Matrix-M malaria vaccine is a simple-to-implement vaccine that can be mass-produced at a low cost, allowing hundreds of millions of doses to be distributed to areas with high malaria burdens.
The Serum Institute of India already has a production capacity of 100 million doses per year, which will be increased over the next two years.
The World Health Organization’s recommendation is essential for UNICEF to obtain and GAVI to purchase the vaccine, clearing the door for vaccination of children in the most vulnerable communities.
The R21/Matrix-MTM malaria vaccine is the conclusion of 30 years of malaria vaccine development at the Jenner Institute at the University of Oxford.
The World Health Organization (WHO) has recommended the use of the R21/Matrix-MTM malaria vaccine produced by the University of Oxford and the Serum Institute of India using Novavax’s adjuvant technology after it met required safety, quality, and effectiveness standards.
The R21/Matrix-MTM malaria vaccine has been recommended for use following a thorough, extensive scientific examination by the WHO’s independent advisory body, the Strategic Advisory Group of Experts (SAGE) and the Malaria Policy Advisory Group (MPAG). The recommendation was based on pre-clinical and clinical trial results that demonstrated good safety and efficacy in four countries with both seasonal and perennial malaria transmission, making it the world’s second-ever WHO recommended vaccine for malaria prevention in children.
The Jenner Institute at Oxford University and the Serum Institute of India produced the vaccine with funding from the European and Developing Countries Clinical Trials Partnership (‘EDCTP’), the Wellcome Trust, and the European Investment Bank (‘EIB’). The R21/Matrix-MTM malaria vaccine is currently approved for use in Ghana, Nigeria, and Burkina Faso. This vaccination, when used in conjunction with public health interventions such as the use of insecticide-treated bed nets, has the potential to save and enhance the lives of millions of children and their families.
The vaccine recently met its major one-year endpoint in a pivotal large-scale Phase III clinical study including 4,800 children from Burkina Faso, Kenya, Mali, and Tanzania, which was primarily sponsored by the Serum Institute of India, with Oxford University serving as the regulatory sponsor. Before publication, the Phase III study data are being peer reviewed.
The vaccine was well accepted and had a favorable safety profile. Using normal age-based administration, the vaccine’s effectiveness over 12 months was 75% (95% CI 71-79; p0.001) at places with high seasonal malaria transmission and 68% (61-74; p0.001) at sites with greater perennial transmission.
Efficacy waned over the first year of follow-up at both seasonal and perennial transmission locations, but a booster dose restored efficacy at seasonal sites, with a vaccination efficacy of 74% (70-77; p0.001).
Vaccine-induced antibody titres were significantly greater in the 5-17-month age group compared to the 18-36-month age group (p0.0001). The younger age group, in which this vaccine is most likely to be widely deployed, demonstrated the highest 12-month vaccine efficacy at both seasonal (79% (73-84, p0.001) and standard (75% (65-83, p0.001) sites.
In a previous Phase IIb clinical trial in Burkina Faso, Oxford researchers and their partners demonstrated that a booster dose of R21/Matrix-M maintained high efficacy against malaria and continued to meet the World Health Organization’s Malaria Vaccine Technology Roadmap goal of at least 75% efficacy. This follows earlier findings from the same trial, which reported 77% effectiveness after one year.
The Phase III findings contribute to a better understanding of how vaccination efficacy varies with age and area in relation to transmission intensity and seasonality. Additional research is being conducted to determine appropriate dose schedules and to track long-term immunological response.
The R21/Matrix-MTM malaria vaccine has been shown to be safe and highly effective across multiple clinical studies, and is now approved as WHO policy for widespread use,’ said Professor Sir Adrian Hill, Director of The Jenner Institute, and Lakshmi Mittal and family Professor of Vaccinology, University of Oxford. The vaccine is quickly deployable, cost effective, and inexpensive, and is ready for distribution in locations where it is most needed, potentially saving hundreds of thousands of lives each year.’
‘The University of Oxford has one of the most active malaria vaccine programs in the world, thanks to a network of global collaborators,’ said Dr Mehreen Datoo, Academic Clinical Fellow in Infectious Diseases & Microbiology at The Jenner Institute, University of Oxford. Today’s accomplishment would not have been possible without the tireless work of our worldwide partners, their wonderful field teams, and, of course, the participants and carers. This is a big step forward in the fight against malaria, but there is still more to be done; we are already developing new vaccine candidates to target other malaria parasites, as well as clinical studies aimed at malaria eradication.’
Dr Lisa Stockdale, Senior Immunologist, The Jenner Institute, University of Oxford said: ‘Today’s news is testament to the work of our dedicated team and means we have another tool with which to fight this disease that kills over half a million people every year. However, further work is critical to establish not just that the vaccine works, but to understand more about how it works, and apply that knowledge to future vaccines.’
Adar Poonawalla, CEO of the Serum Institute of India, said: ‘For far too long, malaria has threatened the lives of billions of people across the globe, disproportionately affecting the most vulnerable amongst us. This is why the WHO recommendation and approval of the R21/Matrix-M™️ vaccine marks a huge milestone on our journey to combat this life-threatening disease, showing what exactly can be achieved when the public and private sector, scientists and researchers, all work together towards a shared goal.
‘As we continue to work together to create a healthier, more equitable world for everyone, I am incredibly proud of the part that the Serum Institute of India has played in developing the R21 malaria vaccine. We look forward to scaling up the vaccine production to ensure that it is accessible to those who need it the most.’
John C. Jacobs, President and Chief Executive Officer, Novavax said: ‘This WHO designation highlights the meaningful contribution that R21/Matrix-M is likely to have in accelerating and expanding access to a safe, efficacious and potentially life-saving vaccine to control malaria – a disease that disproportionately impacts children. Novavax celebrates the importance of this milestone and is proud of the role of our saponin-based Matrix-M adjuvant plays in the R21/Matrix-M malaria vaccine, which has now met the rigorous standards required for WHO recommendation.’
Cost and Production Capacity
The R21/Matrix-MTM vaccine has been licensed to the Serum Institute of India, the world’s largest vaccine maker and a long-term Oxford University partner. Notably, the Serum Institute has already developed a manufacturing capacity of 100 million doses per year, which will be more than doubled in the next two years. This scale of production is significant because vaccination persons at high risk of malaria will be critical in both preventing disease spread and protecting those who have been vaccinated.
Novavax’s unique saponin-based adjuvant, Matrix-M, is licensed to the Serum Institute for use in endemic nations, while Novavax retains commercial rights in non-endemic countries.
Vaccine Distribution
With the WHO’s clearance and recommendations, additional regulatory approvals are expected soon, and R21/Matrix-M vaccine doses might be ready for widespread distribution as early as next year.
more recommended stories
 Iron Deficiency vs Iron Overload in Parkinson’s Disease
Iron Deficiency vs Iron Overload in Parkinson’s DiseaseKey Takeaways (Quick Summary for HCPs).
 Can Ketogenic Diets Help PCOS? Meta-Analysis Insights
Can Ketogenic Diets Help PCOS? Meta-Analysis InsightsKey Takeaways (Quick Summary) A Clinical.
 Silica Nanomatrix Boosts Dendritic Cell Cancer Therapy
Silica Nanomatrix Boosts Dendritic Cell Cancer TherapyKey Points Summary Researchers developed a.
 Vagus Nerve and Cardiac Aging: New Heart Study
Vagus Nerve and Cardiac Aging: New Heart StudyKey Takeaways for Healthcare Professionals Preserving.
 Cognitive Distraction From Conversation While Driving
Cognitive Distraction From Conversation While DrivingKey Takeaways (Quick Summary) Talking, not.
 Fat-Regulating Enzyme Offers New Target for Obesity
Fat-Regulating Enzyme Offers New Target for ObesityKey Highlights (Quick Summary) Researchers identified.
 Spatial Computing Explains How Brain Organizes Cognition
Spatial Computing Explains How Brain Organizes CognitionKey Takeaways (Quick Summary) MIT researchers.
 Gestational Diabetes Risk Identified by Blood Metabolites
Gestational Diabetes Risk Identified by Blood MetabolitesKey Takeaways (Quick Summary for Clinicians).
 Phage Therapy Study Reveals RNA-Based Infection Control
Phage Therapy Study Reveals RNA-Based Infection ControlKey Takeaways (Quick Summary) Researchers uncovered.
 Pelvic Floor Disorders: Treatable Yet Often Ignored
Pelvic Floor Disorders: Treatable Yet Often IgnoredKey Takeaways (Quick Summary) Pelvic floor.

Leave a Comment