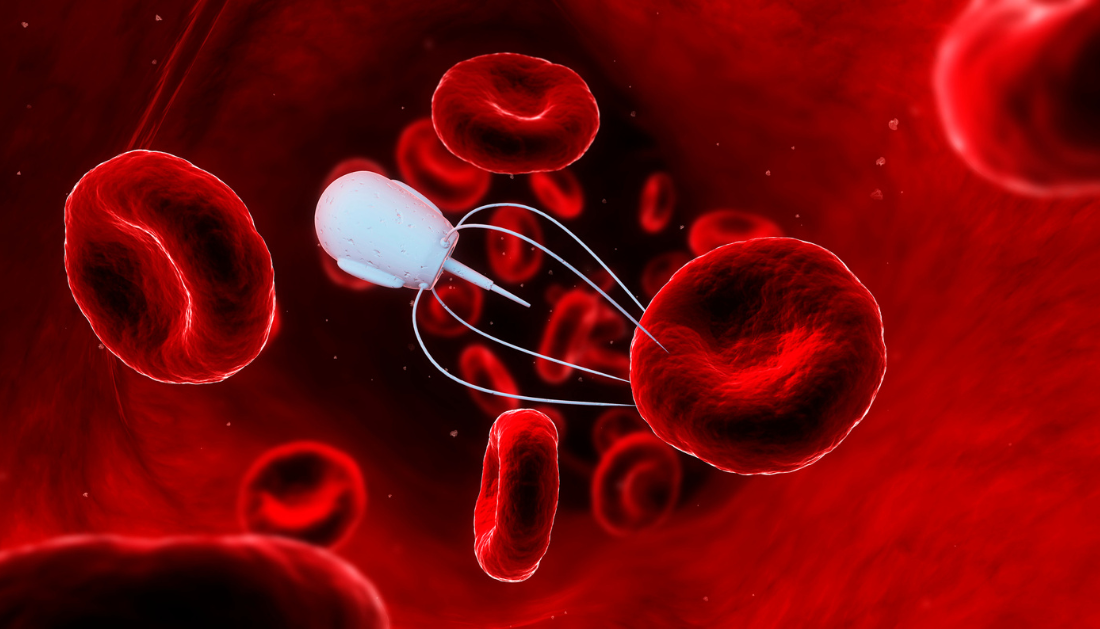

Cancer remains one of the leading causes of morbidity and mortality worldwide. One of the critical challenges in effective cancer treatment is delivering chemotherapy drugs directly into tumor cells, which often develop protective mechanisms to resist drug entry. Recent research has introduced magnetic nanorobots as a potential solution to overcome these barriers and improve drug uptake in tumors.
These spiky nanorobots are engineered to penetrate tumor cell membranes, effectively enhancing chemotherapy efficiency. By physically creating micro-openings in cell membranes, these devices can increase drug concentrations inside cancer cells, thereby improving treatment outcomes and addressing drug resistance. The study, published on August 13, 2025, in Research, demonstrates the potential of magnetic nanorobots in laboratory and animal models.
Explore All Oncology CME/CE Conferences and Online Courses
How Magnetic Nanorobots Enhance Tumor Drug Uptake
The use of magnetic nanorobots leverages both mechanical and magnetic forces to overcome the natural defenses of tumor cells. Cancer cells often resist chemotherapy through rigid membranes and efflux pumps, which expel drugs from the cell before they can exert their therapeutic effect. Traditional nanocarriers, such as liposomes, have improved drug targeting but remain limited by stability, membrane penetration, and controlled drug release.
By contrast, nanorobots are designed with gold nanospikes approximately 500 nanometers wide, coated with nickel to respond to external magnetic fields and titanium to ensure biocompatibility. When guided to tumors using magnetic fields, these nanorobots rotate, mechanically piercing cell membranes to create temporary pores. This process allows chemotherapy drugs, such as doxorubicin, to enter tumor cells directly and more efficiently.
Laboratory Evidence of Efficacy
Laboratory studies using human liver, cervical, and colon cancer cells revealed that nanorobots significantly enhanced drug uptake. Fluorescence imaging confirmed higher intracellular concentrations of chemotherapy agents, with increased application time of the magnetic field correlating with greater drug entry.
Computer simulations of membrane interactions further validated the mechanism, showing that the spiky nanorobots create localized disruptions that increase membrane permeability. Beyond facilitating drug entry, these nanorobots also exert a direct cytotoxic effect termed “mechano-killing,” whereby the mechanical agitation damages tumor cells.
Animal Studies and Clinical Implications
Animal models, specifically mice with liver tumors, demonstrated that combining chemotherapy with magnetic nanorobots resulted in a 61% reduction in tumor growth and 100% survival rate, with minimal side effects. Histological analysis confirmed increased tumor cell death and negligible toxicity to surrounding tissues.
These findings suggest that magnetic nanorobots could serve as an adjunct to traditional chemotherapy, especially in drug-resistant cancers. While the technology is still in early stages, further research could refine delivery methods, assess long-term safety, and explore human clinical applications.
Future Perspectives for Magnetic Nanorobots in Oncology
The introduction of magnetic nanorobots represents a promising advance in nanomedicine and targeted cancer therapy. By physically breaching cancer cell defenses, these devices may enhance the efficacy of existing chemotherapeutics while minimizing systemic toxicity. Future studies will likely focus on optimizing nanorobot design, improving tumor specificity, and integrating imaging guidance for precise clinical applications.
Source:
more recommended stories
 Breast Cancer Prognosis Linked to High-Fat Diet
Breast Cancer Prognosis Linked to High-Fat DietKey Points A high-fat diet accelerated.
 CTNNB1 Syndrome Study Explores Beta-Catenin Defects
CTNNB1 Syndrome Study Explores Beta-Catenin DefectsKey Takeaways Researchers in Spain are.
 Advanced Prostate Cancer and Serial ctDNA Analysis
Advanced Prostate Cancer and Serial ctDNA AnalysisKey Takeaways Serial liquid biopsies using.
 Tuberculosis Breakthrough with Experimental Antibiotics
Tuberculosis Breakthrough with Experimental AntibioticsKey Takeaways Experimental antibiotics disrupt a.
 National Healthy Longevity Trial Receives Federal Support
National Healthy Longevity Trial Receives Federal SupportKey Summary Up to $38 million.
 Red Blood Cells Improve Glucose Tolerance Under Hypoxia
Red Blood Cells Improve Glucose Tolerance Under HypoxiaKey Takeaways for Clinicians Chronic hypoxia.
 Nanoplastics in Brain Tissue and Neurological Risk
Nanoplastics in Brain Tissue and Neurological RiskKey Takeaways for HCPs Nanoplastics are.
 AI Predicts Chronic GVHD Risk After Stem Cell Transplant
AI Predicts Chronic GVHD Risk After Stem Cell TransplantKey Takeaways A new AI-driven tool,.
 Red Meat Consumption Linked to Higher Diabetes Odds
Red Meat Consumption Linked to Higher Diabetes OddsKey Takeaways Higher intake of total,.
 Pediatric Crohn’s Disease Microbial Signature Identified
Pediatric Crohn’s Disease Microbial Signature IdentifiedKey Points at a Glance NYU.

Leave a Comment