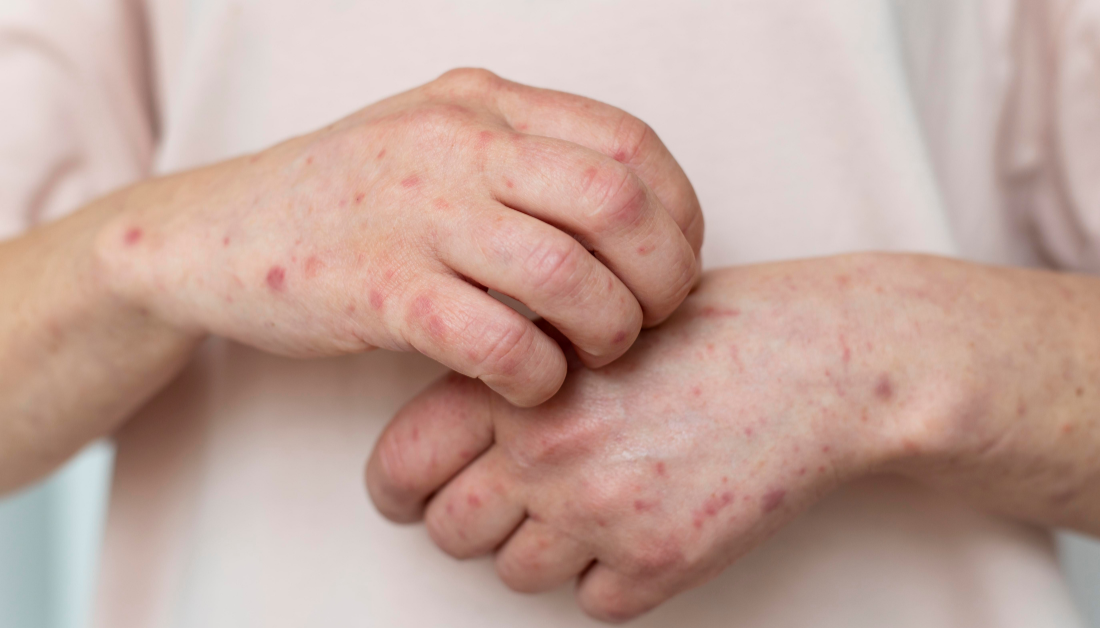

Berdazimer gel 10.3% was approved by the Food and Drug Administration (FDA) on January 5 for the treatment of molluscum contagiosum (MC) in adults and children aged 1 year and older.
Berdazimer, a topical nitric oxide-releasing agent, was approved largely on the basis of B-SIMPLE4, a 12-week pivotal phase 3 trial in which 891 patients with a mean age of 6.6 years (range, 0.9-47.5 years) were randomly assigned to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily. At 12 weeks, 32.4% of patients in the berdazimer group had completely cleared MC lesions, compared to 19.7% in the vehicle group (P.001).
Only 4.1% of berdazimer patients and 0.7% of vehicle patients suffered adverse events that required therapy cessation. Both groups had the most prevalent adverse effects, which were mostly mild or severe application-site discomfort and erythema.
According to a press release issued by Ligand Pharmaceuticals, which acquired berdazimer topical gel from Novan in September 2023, the approval makes berdazimer topical gel 10.3% the first and only topical prescription medication that can be used to treat MC at home, outside of a physician’s office, or outside of other medical settings. Although the mechanism of action of berdazimer for treating molluscum “is unknown,” it has been proven to have antiviral properties.
The medication will be branded as Zelsuvmi and is scheduled to be accessible in the second half of 2024.
On July 21, 2023, the FDA approved a drug-device combination (Ycanth) that contains a formulation of cantharidin solution 0.7% and is administered by healthcare professionals, making topical cantharidin the first approved treatment of MC for adults and pediatric patients aged 2 years or older.
For more information: Novel Drug Approvals for 2024
more recommended stories
 Fentanyl Inhalation: Brain Damage Risks
Fentanyl Inhalation: Brain Damage RisksAfter treating a middle-aged man who.
 Neurocardiac Connectivity in Depression Treatment
Neurocardiac Connectivity in Depression TreatmentHeart rate deceleration and sadness may.
 Antioxidants: Impact on Quality of Life in Acne Vulgaris
Antioxidants: Impact on Quality of Life in Acne VulgarisA recent study published in the.
 Brain Pulsations Linked to High BMI
Brain Pulsations Linked to High BMIAccording to a new study from.
 Brain Age Estimation: EEG Advancements in Neurology
Brain Age Estimation: EEG Advancements in NeurologyTo estimate brain age using EEG.
 Neurodegeneration Linked to Fibrin in Brain Injury
Neurodegeneration Linked to Fibrin in Brain InjuryThe health results for the approximately.
 DELiVR: Advancing Brain Cell Mapping with AI and VR
DELiVR: Advancing Brain Cell Mapping with AI and VRDELiVR is a novel AI-based method.
 Retinal Neurodegeneration in Parkinson’s Disease
Retinal Neurodegeneration in Parkinson’s DiseaseBy measuring the thickness of the.
 Epilepsy Seizures: Role of Astrocytes in Neural Hyperactivity
Epilepsy Seizures: Role of Astrocytes in Neural HyperactivityRoughly 1% of people experience epilepsy.
 Role of Engineered Peptides in Cancer Immunotherapy
Role of Engineered Peptides in Cancer ImmunotherapyIn a recent publication in Nature.

Leave a Comment