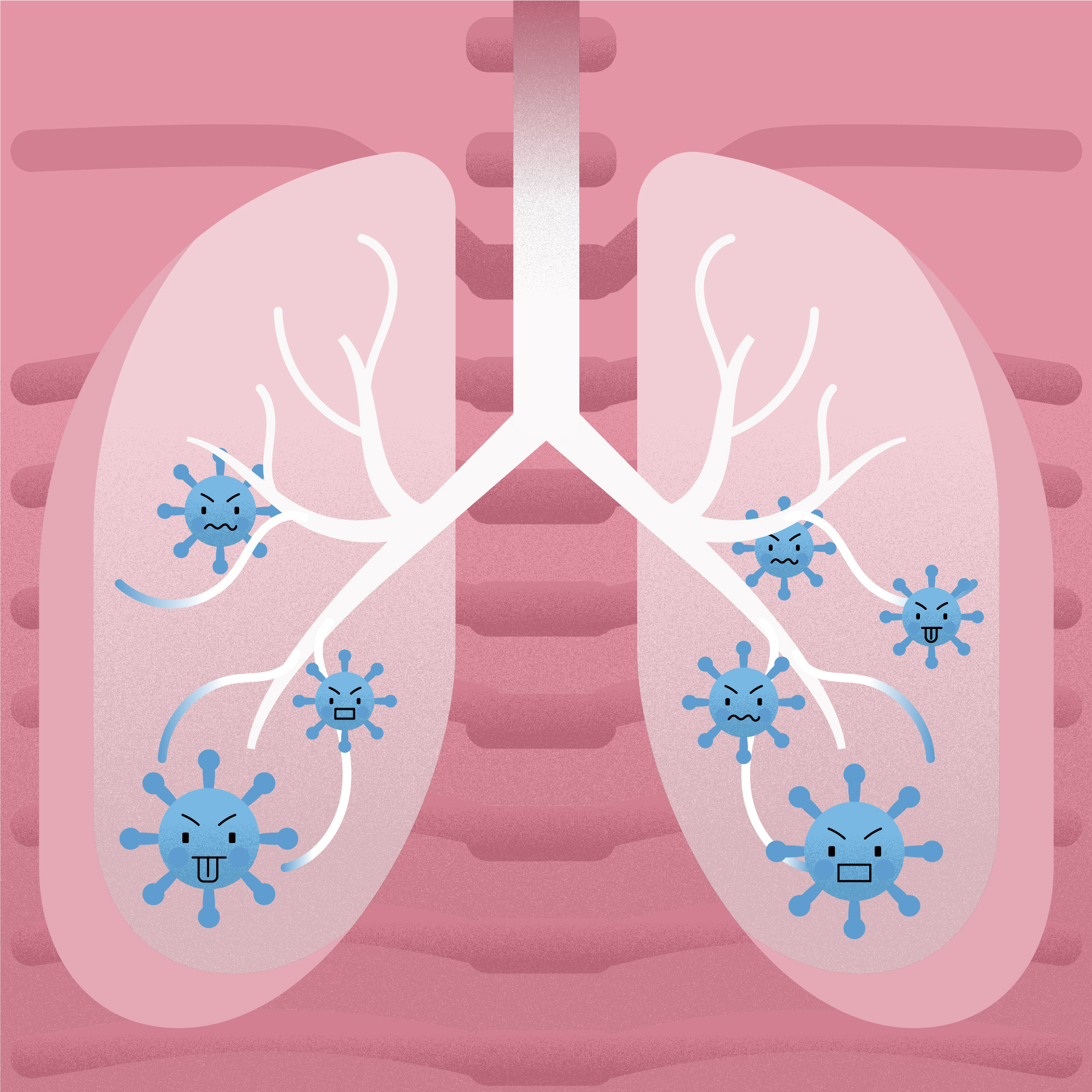

A lung infection known as pneumonia is brought on by bacteria, viruses, or fungi. It is one of the main global causes of illness and mortality, posing a medical, financial, and public health challenge. Different niches are colonized by the microbiome of the human respiratory tract.
Scientists are interested in the respiratory tract microbiome because it promotes immunological function and guards against pathogen infection, which benefits human health. The microbiome composition, pathogen load, and clinical interventions all have an impact on how severe Legionella pneumophila-related bacterial pneumonia is, according to research from the Institut Pasteur and the CNRS.
The journal Cell Reports Medicine published the findings.
This study examined the diversity and makeup of the respiratory tract microbiome (bacteria, archaea, fungi, and protozoa) in patients with pneumonia brought on by the intracellular bacterial pathogen Legionella pneumophila for the duration of their hospitalization. The study was conducted by researchers from the Biology of Intracellular Bacteria Unit at the Institut Pasteur under the direction of Carmen Buchrieser.
Legionnaires’ disease, a severe form of pneumonia caused by L. pneumophila, can be acquired by breathing in infected aerosols from artificial water sources such showers, hot tubs, or air conditioning units. Depending on the clinical setting and the area, the mortality rate for legionnaires’ disease ranges from 5% to 40%. Old age, existing lung diseases, smoking, and immunosuppression are risk factors, and around two thirds of reported cases involve men.
In the European Union, there were confirmed cases of legionnaires’ disease in 4,693 cases in 2005 and 10,004 cases in 2021, a rise of 113%. Climate change, which has led to warmer water temperatures and more frequent and strong floods that enable Legionella to multiply more quickly and enter human surroundings, may be one of the causes of this significant increase.
The research team used a quantification strategy in conjunction with high-throughput bacterial, archaeal, and fungal marker gene sequencing to describe how the respiratory tract microbiome changed in patients over the course of an infection and as a result of hospital-related interventions (like mechanical ventilation and antibiotic administration).
38 hospitalized patients with pneumonia brought on by Legionella pneumophila were studied as a special cohort.
“We discovered complex microbiome dynamics, where commensal and pathogenic microorganisms coexist, and the equilibrium among their abundance drives the microbiome to recovery or dysbiosis,” explains Carmen Buchrieser, lead author of the study and Head of the Institut Pasteur’s Biology of Intracellular Bacteria Unit. The scientists observed that early in hospitalization, microbiome diversity decreased and the pathogen L. pneumophila was killed by the antibiotic treatment.
However, other opportunistic species, frequently resistant to antibiotics, quickly seized the vacant niche. This is a feature that should be taken into account in preventative methods for secondary infections. The lowest diversity and pathogen enrichment were found in the respiratory tract microbiomes with the largest bacterial and fungal loads, suggesting that high biomass could be a biomarker for secondary and/or co-infections.
Finally, the researchers demonstrated a correlation between Legionella biomass and disease severity and comorbidities, indicating that pathogen quantification has to be a part of patient monitoring. Clinical interventions like mechanical ventilation or the use of specific antibiotics have an impact on the microbiome’s composition and, consequently, the course of a disease.
“In this study we also discovered that fungi, archaea and protozoa may be resident and not merely transitory in the respiratory tract of hospitalized patients and that they might contribute to pneumonia progression. This requires further investigation. Our research therefore shows that the interaction between respiratory tract microbiome equilibrium, pathogen load dynamics and clinical interventions plays a crucial role in the recovery of patients with pneumonia,” concludes Carmen Buchrieser.
more recommended stories
 Healthy Habits Slash Diverticulitis Risk in Half: Clinical Insights
Healthy Habits Slash Diverticulitis Risk in Half: Clinical InsightsHealthy Habits Slash Diverticulitis Risk in.
 Caffeine and SIDS: A New Prevention Theory
Caffeine and SIDS: A New Prevention TheoryFor the first time in decades,.
 Microbial Metabolites Reveal Health Insights
Microbial Metabolites Reveal Health InsightsThe human body is not just.
 Reelin and Cocaine Addiction: A Breakthrough Study
Reelin and Cocaine Addiction: A Breakthrough StudyA groundbreaking study from the University.
 Preeclampsia and Stroke Risk: Long-Term Effects
Preeclampsia and Stroke Risk: Long-Term EffectsPreeclampsia (PE) – a hypertensive disorder.
 Statins and Depression: No Added Benefit
Statins and Depression: No Added BenefitWhat Are Statins Used For? Statins.
 Azithromycin Resistance Rises After Mass Treatment
Azithromycin Resistance Rises After Mass TreatmentMass drug administration (MDA) of azithromycin.
 Generative AI in Health Campaigns: A Game-Changer
Generative AI in Health Campaigns: A Game-ChangerMass media campaigns have long been.
 Molecular Stress in Aging Neurons Explained
Molecular Stress in Aging Neurons ExplainedAs the population ages, scientists are.
 Higher BMI and Hypothyroidism Risk Study
Higher BMI and Hypothyroidism Risk StudyA major longitudinal study from Canada.

Leave a Comment