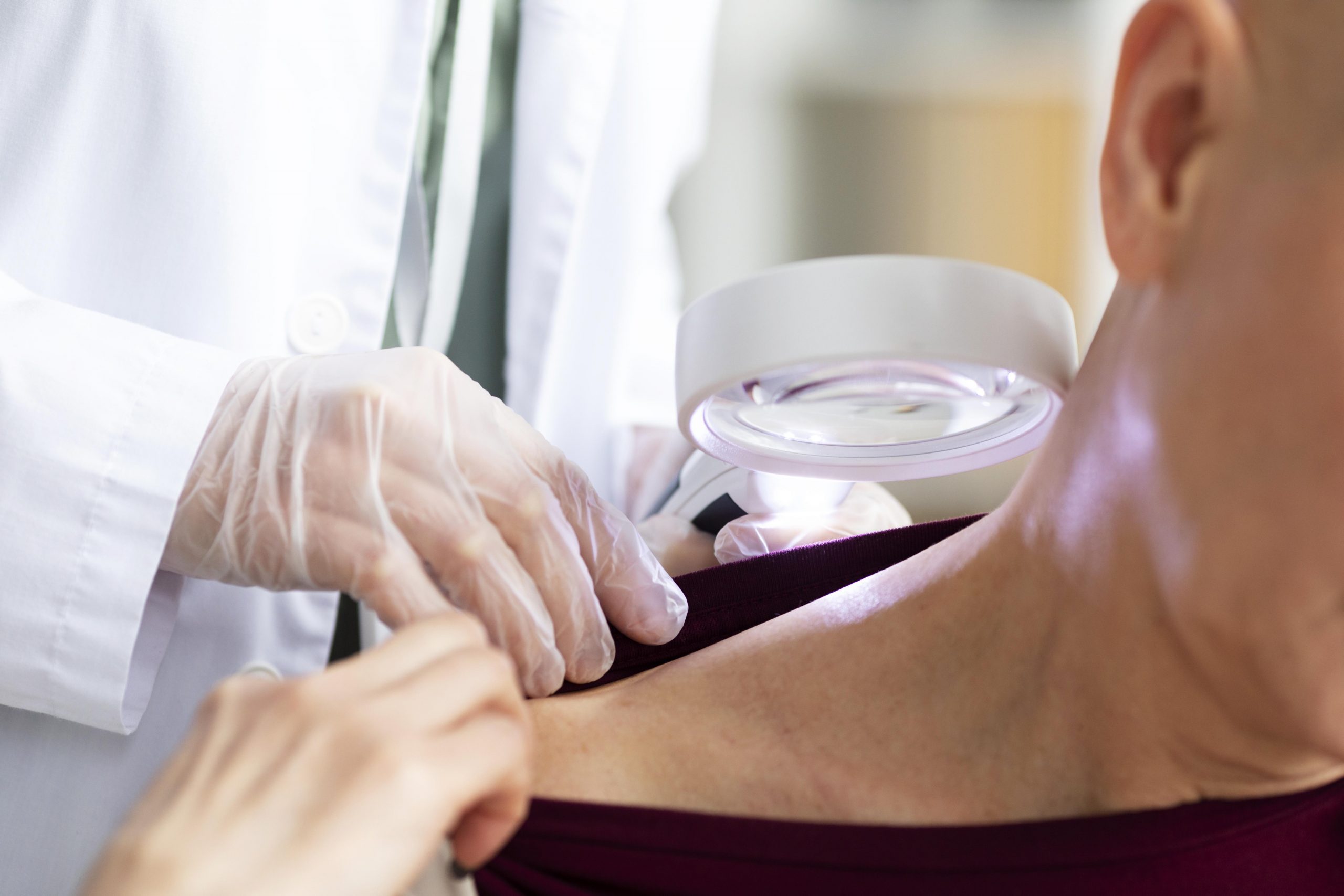

According to studies conducted by UCLA Jonsson Comprehensive Cancer Center researchers, patients with advanced melanoma responded differently to PD-1 checkpoint blockade immunotherapy depending on a number of variables, including whether or not they had previously received CTLA-4 blockade, another type of immunotherapy.
They published their findings in Cancer Cell after analyzing seven data sets collected over the previous ten years that comprised the tumor biopsy results of more than 500 patients.
“In our large set of data, features that have been used to predict response to anti-PD-1 checkpoint blockade therapy—often called biomarkers –related to the presence of certain immune cell types in the tumor and the genetic profile of the tumors themselves were modified by a patient’s treatment history,” said lead author Katie Campbell, Ph.D., a postdoctoral fellow in hematology/oncology at UCLA Jonsson Comprehensive Cancer Center.
Immune therapy including anti-PD-1 blockade and anti-CTLA-4 blockage are frequently used to treat patients who have been diagnosed with metastatic melanoma, either in combination or separately. These checkpoint inhibitors improve the body’s immune response to cancer by blocking certain proteins that reduce the potency of T cells.
“As translational scientists, when we work with clinicians, one of the goals is to think about how biomarkers can be used to inform clinical benefit. If we can predict which patients are or are not going to respond to therapy from studying their biopsies, we can start to more strategically define which therapies or combinations of therapies should be used and when,
“Since the current treatment paradigm for melanoma involves combinations or sequential use of immune checkpoint therapies, our study supports how these therapies may work together to effectively treat melanoma. It also highlights the importance of a patient’s prior treatment history as a modifying factor to consider when planning a treatment strategy,” said co-senior author Dr. Antoni Ribas, director of the Tumor Immunology Program at UCLA Jonsson Comprehensive Cancer Center and the Parker Institute for Cancer Immunotherapy Center at UCLA.
The majority of studies on biomarkers and criteria affecting success are based on limited series of samples, despite the fact that immunotherapy is increasingly used in the treatment of late-stage tumors. In order to discover important variables linked to treatment response, the multidisciplinary and multicenter research team set out to compile and harmonize a huge set of tumor and clinical data from melanoma patients.
“The cohesive processing of clinical data sets requires collaboration among experts with knowledge in computer science, statistics, biology, immunotherapy, informatics, and translational and clinical medicine. We undertook this project to establish a resource for other researchers, with the goal of identifying statistically significant correlates of melanoma responses to anti-PD-1 therapy,” Campbell said.
“As we performed the analyses, the greatest differences were seen when we accounted for a patient’s prior treatment with the anti-CTLA-4 blockade,” she added. “the context in which a biopsy is collected needs to be considered to better define how biomarkers should be implemented in the clinical setting.”
The researchers claimed that they were able to account for some of the vast disparities across patients, tumors, and treatment histories by processing the DNA and RNA sequencing data from hundreds of patients on a single, integrated pipeline. They also took into account clinical characteristics that may be crucial for figuring out whether a patient responded to anti-PD-1 blocking medication or not.
The findings offer a framework and a road map, even though they do not precisely describe how or when to use the biomarker information in clinical settings.
Christine Spencer and Daniel Wells from the Parker Institute for Cancer Immunotherapy in San Francisco are co-senior authors in addition to lead author Campbell and co-senior author Ribas, who are both corresponding authors. Together with Meelad Amouzgar of Stanford University and the Parker Institute for Cancer Immunotherapy, Campbell co-led this study.
more recommended stories
 T-bet and the Genetic Control of Memory B Cell Differentiation
T-bet and the Genetic Control of Memory B Cell DifferentiationIn a major advancement in immunology,.
 Ultra-Processed Foods May Harm Brain Health in Children
Ultra-Processed Foods May Harm Brain Health in ChildrenUltra-Processed Foods Linked to Cognitive and.
 Parkinson’s Disease Care Advances with Weekly Injectable
Parkinson’s Disease Care Advances with Weekly InjectableA new weekly injectable formulation of.
 Brain’s Biological Age Emerges as Key Health Risk Indicator
Brain’s Biological Age Emerges as Key Health Risk IndicatorClinical Significance of Brain Age in.
 Children’s Health in the United States is Declining!
Children’s Health in the United States is Declining!Summary: A comprehensive analysis of U.S..
 Autoimmune Disorders: ADA2 as a Therapeutic Target
Autoimmune Disorders: ADA2 as a Therapeutic TargetAdenosine deaminase 2 (ADA2) has emerged.
 Is Prediabetes Reversible through Exercise?
Is Prediabetes Reversible through Exercise?150 Minutes of Weekly Exercise May.
 New Blood Cancer Model Unveils Drug Resistance
New Blood Cancer Model Unveils Drug ResistanceNew Lab Model Reveals Gene Mutation.
 Healthy Habits Slash Diverticulitis Risk in Half: Clinical Insights
Healthy Habits Slash Diverticulitis Risk in Half: Clinical InsightsHealthy Habits Slash Diverticulitis Risk in.
 Caffeine and SIDS: A New Prevention Theory
Caffeine and SIDS: A New Prevention TheoryFor the first time in decades,.

Leave a Comment