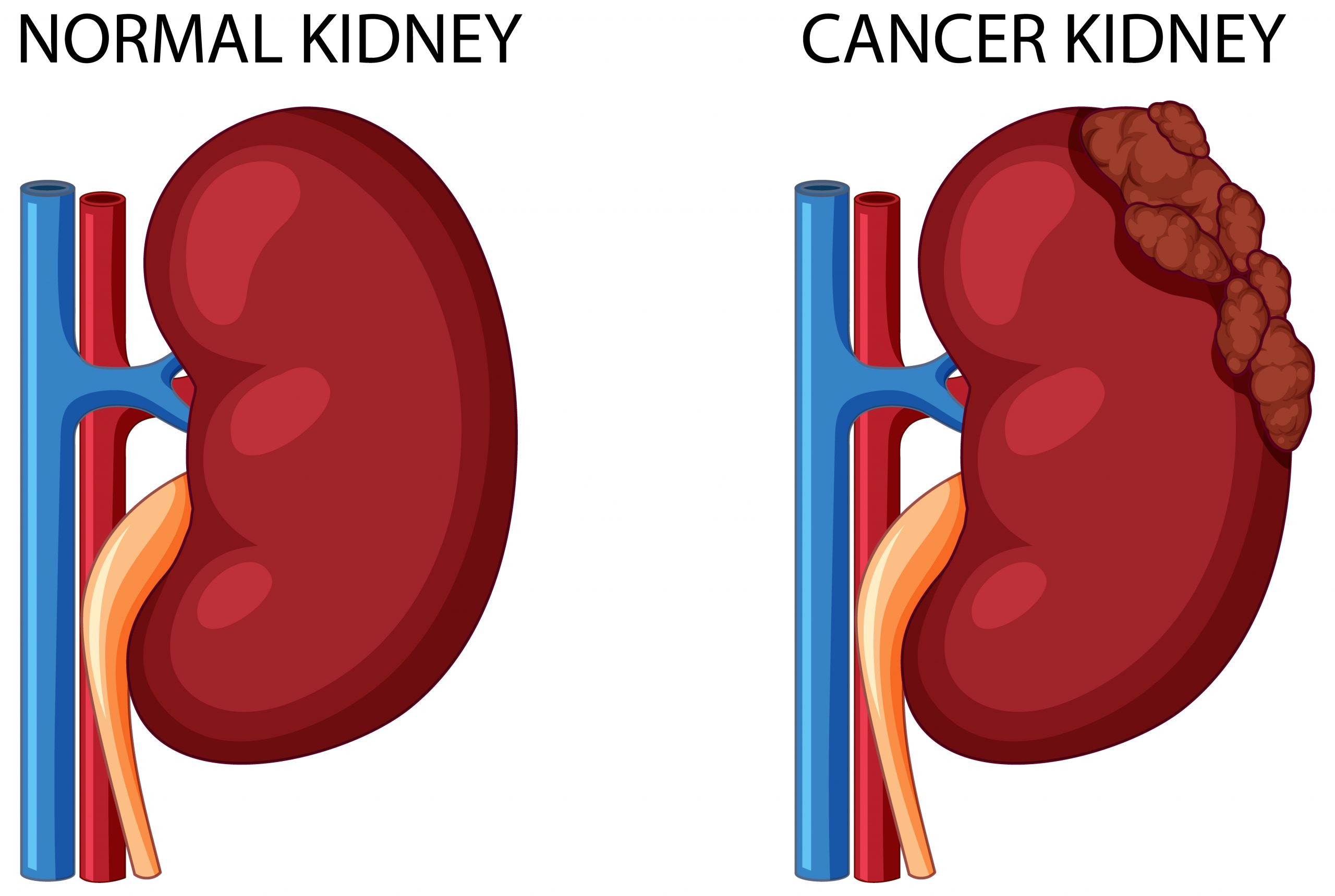

A new model of aggressive renal cell carcinoma (RCC) has been developed by researchers at The University of Texas MD Anderson Cancer Center, revealing molecular mechanisms and genomic events that cause chromosomal instability and drive metastatic kidney cancer.
The findings, published in Nature Cancer, show that the deletion of a cluster of interferon receptor (IFNR) genes is critical in cancer cells becoming tolerant to chromosomal instability. This genomic trait could aid clinicians in predicting a tumor’s proclivity to spread and develop resistant to treatment.
Researchers led by Luigi Perelli, M.D., Ph.D., postdoctoral fellow in Genitourinary Medical Oncology, and Giannicola Genovese, M.D., Ph.D., professor in Genitourinary Medical Oncology, used CRISPR/Cas9 gene editing to create a model that faithfully represents RCC in humans, with cross-species analyses providing additional insights into the mechanisms involved in aggressive kidney cancer evolution.
“Until now, there haven’t been effective experimental models for metastatic renal cancer progression, but we introduced specific mutations that closely mimic the early stages of human cancers to see how tumors evolve and metastasize,” Genovese said. “These tumors become extremely genomically unstable, and, to tolerate this instability, they tend to lose genetic material at a specific site where the interferon genes are located. These insights can help clinicians identify tumors that have the genomic potential to become aggressive.”
Renal cell carcinoma is the most prevalent type of kidney cancer, and patients are often successfully treated with surgery, targeted therapy, immunotherapy, or a combination of these therapies. However, up to one-third of these individuals will experience severe disease progression, emphasizing the importance of understanding particular pathways that promote metastasis in order to discover more effective therapeutic options and anticipate treatment responses.
Cancer is characterized by chromosomal instability, which is linked to resistance to various types of therapy and a bad prognosis. However, it is unknown if specific types of chromosomal aberrations have a role in metastasis and how cancers tolerate them.
The researchers created RCC models lacking common tumor suppressor genes using CRISPR/Cas 9-based genome editing. They then used cell cycle regulator genes to imitate a common chromosomal defect associated with metastatic RCC in people, resulting in a phenotype that matched the human illness. This is the first immunocompetent somatic mosaic model for metastatic RCC, which means that the animal has a collection of various mutations that result in uncontrolled cell growth while yet retaining a functional immune system.
The researchers discovered molecular drivers of RCC and developed a novel understanding of the evolution of chromosomal instability by using genome sequencing and single-cell RNA sequencing to further investigate these models.
Their single cell investigations revealed that the model repressed a cluster of highly conserved IFNR genes, and that this cluster ordinarily operates as a crucial gatekeeper, or tumor suppressor, of renal cancer growth.
IFNR gene clusters are generally implicated in immune responses. The researchers observed an inverse association between the absence of these IFNR genes and aneuploidy, a disorder characterised by an aberrant number of chromosomes, after evaluating numerous data sets from both mice and humans.
According to the findings, cancers adapt to high levels of chromosomal instability by disrupting the IFNR pathway, and this is most likely a primary biomarker of metastatic potential. It also demonstrates how renal malignancies in other animals have followed similar evolutionary paths that converge around chromosomal instability, which may explain the tumor’s variety.
The researchers intend to test drug combinations in these newly produced models in the future to understand how tumors adapt to various therapy, with the objective of quickly transferring these studies into clinical trials that can assist predict treatment response in RCC patients.
more recommended stories
 Iron Deficiency vs Iron Overload in Parkinson’s Disease
Iron Deficiency vs Iron Overload in Parkinson’s DiseaseKey Takeaways (Quick Summary for HCPs).
 Can Ketogenic Diets Help PCOS? Meta-Analysis Insights
Can Ketogenic Diets Help PCOS? Meta-Analysis InsightsKey Takeaways (Quick Summary) A Clinical.
 Silica Nanomatrix Boosts Dendritic Cell Cancer Therapy
Silica Nanomatrix Boosts Dendritic Cell Cancer TherapyKey Points Summary Researchers developed a.
 Vagus Nerve and Cardiac Aging: New Heart Study
Vagus Nerve and Cardiac Aging: New Heart StudyKey Takeaways for Healthcare Professionals Preserving.
 Cognitive Distraction From Conversation While Driving
Cognitive Distraction From Conversation While DrivingKey Takeaways (Quick Summary) Talking, not.
 Fat-Regulating Enzyme Offers New Target for Obesity
Fat-Regulating Enzyme Offers New Target for ObesityKey Highlights (Quick Summary) Researchers identified.
 Spatial Computing Explains How Brain Organizes Cognition
Spatial Computing Explains How Brain Organizes CognitionKey Takeaways (Quick Summary) MIT researchers.
 Gestational Diabetes Risk Identified by Blood Metabolites
Gestational Diabetes Risk Identified by Blood MetabolitesKey Takeaways (Quick Summary for Clinicians).
 Phage Therapy Study Reveals RNA-Based Infection Control
Phage Therapy Study Reveals RNA-Based Infection ControlKey Takeaways (Quick Summary) Researchers uncovered.
 Pelvic Floor Disorders: Treatable Yet Often Ignored
Pelvic Floor Disorders: Treatable Yet Often IgnoredKey Takeaways (Quick Summary) Pelvic floor.

Leave a Comment