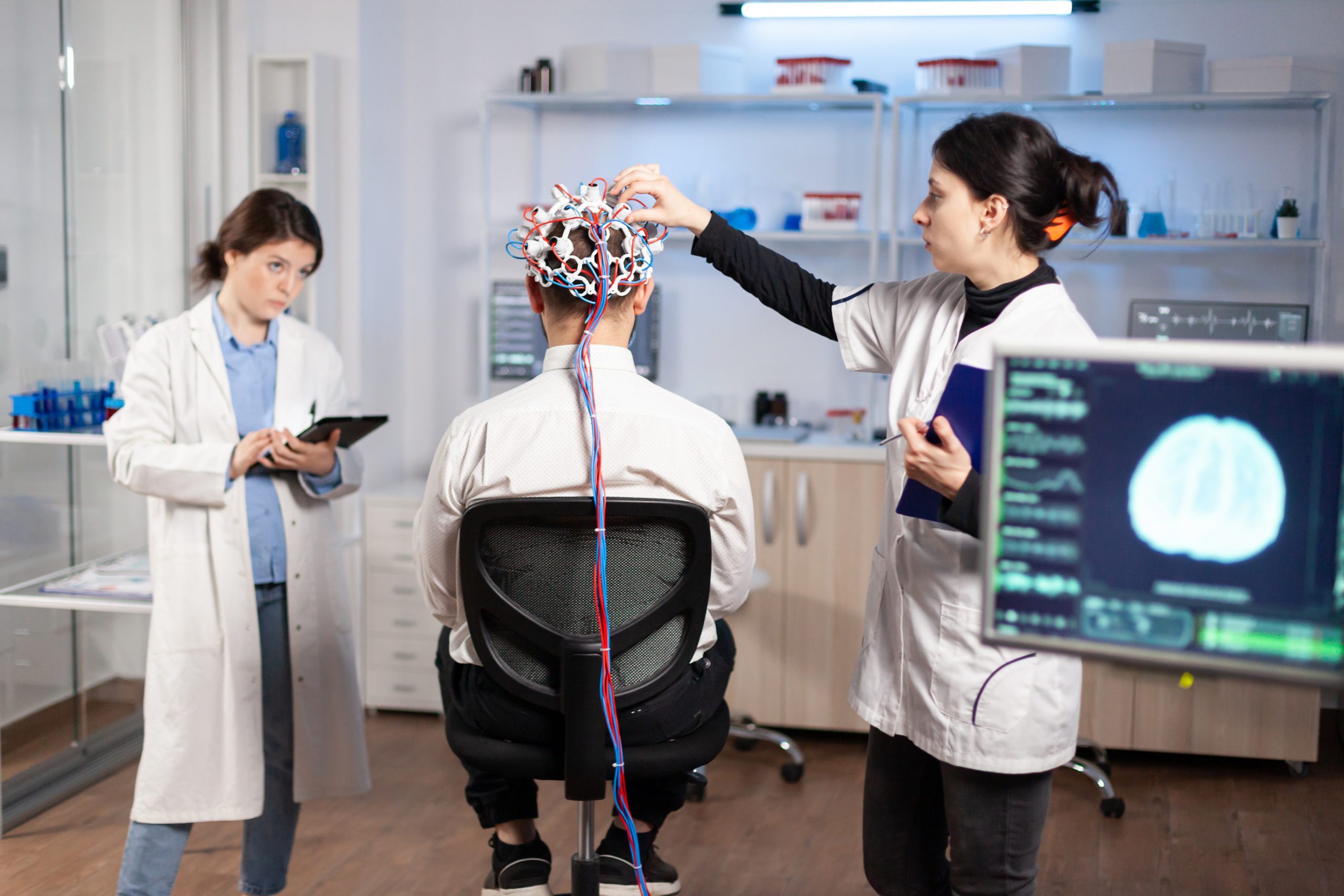

Why do some people develop Alzheimer’s disease while others do not? And, even more perplexing, why do many people with toxic amyloid aggregates in their brains—a telltale marker of Alzheimer’s brain pathology—never get Alzheimer’s-related dementias?
Researchers from the University of Pittsburgh School of Medicine appear to have discovered the solution. According to new research published today (May 29) in Nature Medicine, star-shaped brain cells called astrocytes are critical in swaying the pendulum in Alzheimer’s disease progression.
The Pitt-led research team discovered that only those with a combination of amyloid burden and blood markers of abnormal astrocyte activation, or reactivity, would progress to symptomatic Alzheimer’s in the future, a critical discovery for drug development aimed at halting progression.
Our study argues that testing for the presence of brain amyloid along with blood biomarkers of astrocyte reactivity is the optimal screening to identify patients who are most at risk for progressing to Alzheimer’s disease,” said senior author Tharick Pascoal, M.D., Ph.D., associate professor of psychiatry and neurology at Pitt. “This puts astrocytes at the center as key regulators of disease progression, challenging the notion that amyloid is enough to trigger Alzheimer’s disease.”
Alzheimer’s disease is a neurological illness that causes progressive memory loss and dementia, depriving people of many useful years of their lives. The formation of amyloid plaques—protein aggregates stuck between nerve cells of the brain—and clumps of disordered protein fibers, known as tau tangles, accumulating inside neurons is the hallmark of Alzheimer’s disease at the tissue level.
For many decades, brain scientists believed that amyloid plaques and tau tangles were not merely an indication of Alzheimer’s disease, but also the disease’s direct cause. This notion also encouraged pharma companies to invest substantially in compounds that target amyloid and tau, ignoring the significance of other brain functions, such as the neuroimmune system.
Recent findings by labs like Pascoal’s imply that disturbance of other brain processes, such as increased brain inflammation, may be just as significant in initiating the catastrophic cascade of neuronal death that leads rapid cognitive loss as amyloid burden itself.
Pascoal and his colleagues previously discovered that brain tissue inflammation promotes the spread of pathologically misfolded proteins in the brain and is a direct cause of eventual cognitive impairment in Alzheimer’s disease patients. Researchers have shown that cognitive decline can be predicted by a blood test almost two years later.
Astrocytes are specialized cells found in abundance in brain tissue. As with other glia—brain’s resident immune cells—astrocytes provide nourishment and oxygen to neuronal cells while also defending them from pathogens. However, because glial cells can not transmit electricity and did not appear to have a direct part in how neurons interact with one another at first, their role in health and sickness was missed. Pitt’s most recent study changes that.
“Astrocytes coordinate brain amyloid and tau relationship like a conductor directing the orchestra,” said lead author of the study Bruna Bellaver, Ph.D., postdoctoral associate at Pitt. “This can be a game-changer to the field, since glial biomarkers in general are not considered in any main disease model.”
Blood samples from three different investigations of cognitively unimpaired elderly persons were evaluated for astrocyte reactivity biomarkers—glial fibrillary acidic protein, or GFAP—as well as the presence of pathogenic tau. Only individuals who were positive for both amyloid and astrocyte reactivity exhibited indications of progressively growing tau pathology, indicating a tendency to clinical symptoms of Alzheimer’s disease, according to the study.
The findings have immediate implications for future Alzheimer’s medication candidate clinical trials. Trials are going to earlier and earlier phases of pre-symptomatic disease in order to arrest disease development sooner, making accurate early detection of Alzheimer’s risk vital for success. Because a considerable proportion of amyloid-positive individuals do not advance to clinical manifestations of Alzheimer’s, amyloid positivity alone does not establish an individual’s eligibility for therapy.
The inclusion of astrocyte reactivity markers, such as GFAP, in the panel of diagnostic tests will allow for better selection of patients who are likely to progress to later stages of Alzheimer’s disease and, as a result, help fine-tune selection of candidates for therapeutic interventions who are more likely to benefit.
more recommended stories
 Is Prediabetes Reversible through Exercise?
Is Prediabetes Reversible through Exercise?150 Minutes of Weekly Exercise May.
 New Blood Cancer Model Unveils Drug Resistance
New Blood Cancer Model Unveils Drug ResistanceNew Lab Model Reveals Gene Mutation.
 Healthy Habits Slash Diverticulitis Risk in Half: Clinical Insights
Healthy Habits Slash Diverticulitis Risk in Half: Clinical InsightsHealthy Habits Slash Diverticulitis Risk in.
 Caffeine and SIDS: A New Prevention Theory
Caffeine and SIDS: A New Prevention TheoryFor the first time in decades,.
 Microbial Metabolites Reveal Health Insights
Microbial Metabolites Reveal Health InsightsThe human body is not just.
 Reelin and Cocaine Addiction: A Breakthrough Study
Reelin and Cocaine Addiction: A Breakthrough StudyA groundbreaking study from the University.
 Preeclampsia and Stroke Risk: Long-Term Effects
Preeclampsia and Stroke Risk: Long-Term EffectsPreeclampsia (PE) – a hypertensive disorder.
 Statins and Depression: No Added Benefit
Statins and Depression: No Added BenefitWhat Are Statins Used For? Statins.
 Azithromycin Resistance Rises After Mass Treatment
Azithromycin Resistance Rises After Mass TreatmentMass drug administration (MDA) of azithromycin.
 Generative AI in Health Campaigns: A Game-Changer
Generative AI in Health Campaigns: A Game-ChangerMass media campaigns have long been.

Leave a Comment