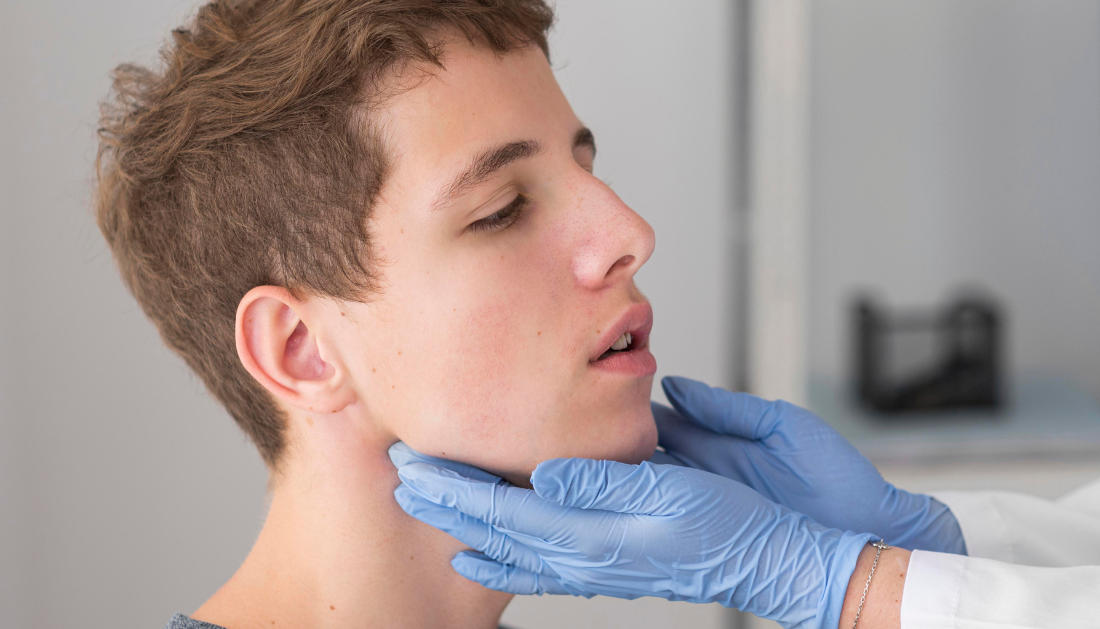

A recent study published in Emerging Infectious Diseases investigated the potential link between COVID-19 immunization and facial palsy (FP). They discovered an elevated incidence of FP after 28 days of immunization, particularly following the first and second doses of both messenger ribonucleic acid (mRNA) and viral vaccinations.
Background
During the COVID-19 epidemic, immunizations were distributed quickly, generating worries about potential side effects, including FP. Although no serious safety issues arose during clinical trials, there was an imbalance of FP instances among vaccinated persons compared to the general population.
FP, which causes rapid facial muscular paralysis, has been associated to infections, autoimmune reactions, and immunization. The Safety Platform for Emergency Vaccines (SPEAC) prioritized it as an adverse event. Several research have looked into the link between COVID-19 vaccines and FP, but the findings have been conflicting.
Differences in research population sizes, ethnicities, vaccination types, doses, and statistical methodologies could explain the disparities in results. Even a systematic review and meta-analysis of these studies were unable to adequately settle the issue due to the narrow inclusion criteria.
The contradictory findings highlight the need for more robust, large-scale studies to develop a clear consensus on the safety of COVID-19 vaccinations for FP. In the current investigation, researchers used a self-controlled case series (SCCS) analysis to determine the potential relationship between FP and COVID-19 vaccinations.
About the study
The current investigation was conducted by the COVID-19 Vaccine Safety Research Committee (CoVaSC) in South Korea. Researchers used two big databases—the COVID-19 immunization registry and National Health Insurance Service (NHIS) claims data—to identify individuals over the age of 18 who received COVID-19 vaccines between February 2021 and March 2022.
Individuals with missing immunization records and a past FP diagnosis were excluded. Vaccines were divided into three types: mRNA vaccines (BNT162b2 and mRNA-1273), viral vector vaccines (ChAdOx1 nCoV-19 and Ad26.COV2.S), and recombinant protein vaccines (NVX-CoV2373).
The SCCS design was used to examine FP incidence between a 28-day post-vaccination risk window and a control window. Age, gender, comorbidities, and insurance status were all taken into consideration. The primary outcome was a diagnosis of FP followed by an oral or parenteral corticosteroid prescription on the same day. The study sought to investigate vaccine kinds, dosages, and homologous or heterologous vaccination regimens.
T-tests, chi-square tests, conditional Poisson regression, incidence rate ratios (IRRs), subgroup analyses, and Benjamini-Hochberg adjustment were all used in the statistical study. To guarantee robustness, sensitivity and subgroup analyses were also carried out.
In conclusion
Regardless of vaccination type or dosage, the study concluded that there was a transient increase in the risk of FP after receiving any dose of the COVID-19 vaccine.
The actual number of cases was low, though, and since FP is usually mild and treatable, this risk shouldn’t discourage vaccination. Doctors are urged to talk with patients about the risk-benefit ratio of COVID-19 vaccinations and to keep an eye out for neurological symptoms after vaccination.
For more information: Risk for facial palsy after COVID-19 vaccination, South Korea, 2021–2022, Emerging Infectious Diseases, doi: 10.3201/eid3011.240610
more recommended stories
 New Blood Cancer Model Unveils Drug Resistance
New Blood Cancer Model Unveils Drug ResistanceNew Lab Model Reveals Gene Mutation.
 Healthy Habits Slash Diverticulitis Risk in Half: Clinical Insights
Healthy Habits Slash Diverticulitis Risk in Half: Clinical InsightsHealthy Habits Slash Diverticulitis Risk in.
 Caffeine and SIDS: A New Prevention Theory
Caffeine and SIDS: A New Prevention TheoryFor the first time in decades,.
 Microbial Metabolites Reveal Health Insights
Microbial Metabolites Reveal Health InsightsThe human body is not just.
 Reelin and Cocaine Addiction: A Breakthrough Study
Reelin and Cocaine Addiction: A Breakthrough StudyA groundbreaking study from the University.
 Preeclampsia and Stroke Risk: Long-Term Effects
Preeclampsia and Stroke Risk: Long-Term EffectsPreeclampsia (PE) – a hypertensive disorder.
 Statins and Depression: No Added Benefit
Statins and Depression: No Added BenefitWhat Are Statins Used For? Statins.
 Azithromycin Resistance Rises After Mass Treatment
Azithromycin Resistance Rises After Mass TreatmentMass drug administration (MDA) of azithromycin.
 Generative AI in Health Campaigns: A Game-Changer
Generative AI in Health Campaigns: A Game-ChangerMass media campaigns have long been.
 Molecular Stress in Aging Neurons Explained
Molecular Stress in Aging Neurons ExplainedAs the population ages, scientists are.

Leave a Comment