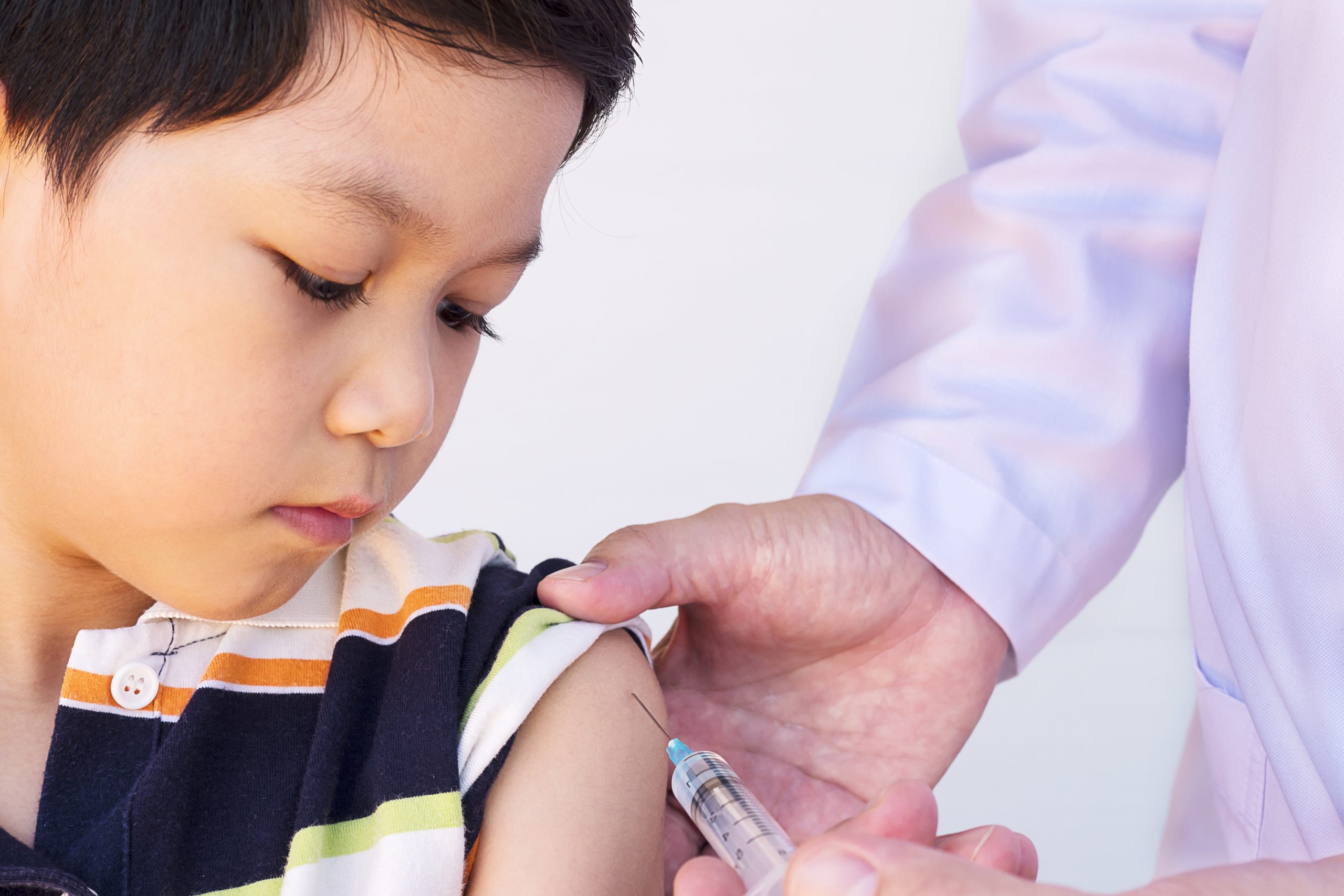

A recent study shows that children with type 1 diabetes between the ages of 2 and 6 benefit from the use of an artificial pancreas that was first created at the University of Virginia Center for Diabetes Technologies. The New England Journal of Medicine has published the specifics of the clinical research as well as its conclusions.
Comparing trial participants utilizing the artificial pancreas to participants in a control group who continued to rely on the methods they were currently using to manage their blood sugar, the artificial pancreas group spent almost three more hours per day in their target blood sugar range.
An automated blood glucose monitor and controller, the Tandem Diabetes Care Control-IQ system is a diabetes management tool.
The artificial pancreas features an insulin pump that can regulate the insulin dose based on data from the patient’s glucose monitoring device. The approach has already been accepted by the U.S. Food and Drug Administration for persons with type 1 diabetes ages 6 and older based on findings from two earlier trials.
“After the resounding success of Control-IQ technology in people ages 6 and up, it is very rewarding to see our youngest patients, and often the most challenging patients to help, benefit as well,” said Marc D. Breton, Ph.D., a UVA School of Medicine researcher who served as the trial’s principal investigator and was recently honored as UVA’s 2022 Innovator of the Year. “With these results, we have now accumulated years of clinical validation of this system across all age groups and look forward to seeing this life-changing technology made available to the broadest possible population.”
102 kids between the ages of 2 and 6 were enrolled in the study at UVA, Stanford University, and the University of Colorado, and 68 of them were randomly assigned to utilize an artificial pancreas system for 13 weeks while the other 34 kids were placed in the control group. Throughout the study, every participant continued to go about their daily lives as usual.
Within their target blood glucose range, participants using the artificial pancreas spent on average roughly 12 percentage points more time than those in the control group did overall, and 18 percentage points more time between the hours of 10 p.m. and 6 a.m. Controlling blood sugar levels at night is crucial because severe, untreated hypoglycemia (extremely low blood sugar levels) can cause seizures, coma, or even death.
Participants were able to utilize the artificial pancreas safely overall, the researchers discovered. In the artificial pancreas group, there were two incidences of severe hypoglycemia, compared to one in the control group. In the artificial pancreas group, there was also one instance of diabetic ketoacidosis brought on by a broken thin plastic line connecting the insulin pump to the patient’s body.
What’s noteworthy is that more than 90% of the visits for the trial were virtual, including 80% of the training sessions for the artificial pancreas. The technology’s simplicity of usage and its promise for regions without convenient access to endocrinologists are highlighted by the stated outcomes being achieved under these circumstances.
“At the end of the day, this technology significantly improved glycemia and ensured the safety of our youngest patients, but perhaps just as importantly it lessened these families’ constant anxiety about glucose levels, especially during the night,” Breton said. “It is incredibly rewarding for us to hear about these families’ experiences and how they manage to integrate these new tools in their life, offering some reprieve to the challenges they face.”
The study’s authors are R. Paul Wadwa, Zachariah W. Reed, Bruce A. Buckingham, Mark D. DeBoer, Laya Ekhlaspour, Gregory P. Forlenza, Melissa Schoelwer, John Lum, Craig Kollman, Roy W. Beck, and Breton.
more recommended stories
 New Blood Cancer Model Unveils Drug Resistance
New Blood Cancer Model Unveils Drug ResistanceNew Lab Model Reveals Gene Mutation.
 Osteoarthritis Genetics Study Uncovers New Treatment Hope
Osteoarthritis Genetics Study Uncovers New Treatment HopeOsteoarthritis- the world’s leading cause of.
 Antibody Breakthrough in Whooping Cough Vaccine
Antibody Breakthrough in Whooping Cough VaccineWhooping cough vaccine development is entering.
 Scientists Unveil Next-Gen Eye-Tracking with Unmatched Precision
Scientists Unveil Next-Gen Eye-Tracking with Unmatched PrecisionEye-tracking technology has long been a.
 Men5CV: Hope for Ending Africa’s Meningitis Epidemics
Men5CV: Hope for Ending Africa’s Meningitis EpidemicsA landmark global health study led.
 Stem Cell Therapy Shows 92% Success in Corneal Repair
Stem Cell Therapy Shows 92% Success in Corneal RepairA groundbreaking stem cell therapy known.
 Gene Therapy for Maple Syrup Urine Disease
Gene Therapy for Maple Syrup Urine DiseaseResearchers at UMass Chan Medical School.
 How Fast Are Your Organs Aging? Simple Blood Test May Tell
How Fast Are Your Organs Aging? Simple Blood Test May TellNew research from University College London.
 HEALEY Platform Accelerates ALS Therapy Research
HEALEY Platform Accelerates ALS Therapy ResearchA New Era of ALS Clinical.
 Low-Oxygen Therapy in a HypoxyStat Pill? Scientists Say It’s Possible
Low-Oxygen Therapy in a HypoxyStat Pill? Scientists Say It’s PossibleA New Approach to Oxygen Regulation-HypoxyStat.

Leave a Comment