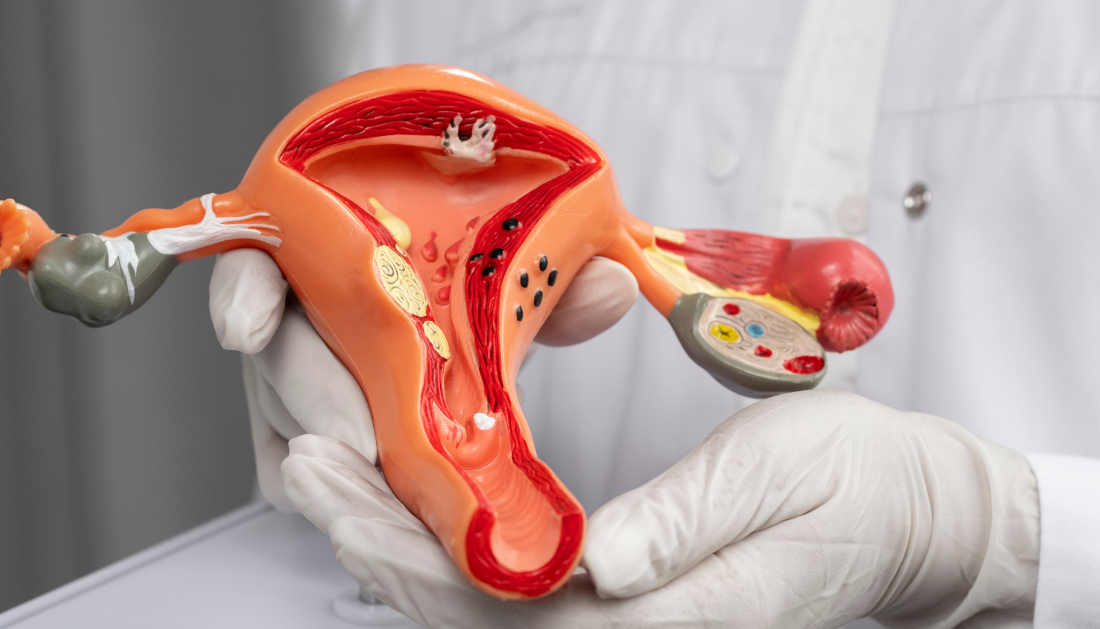

In a recent study published in eBioMedicine, researchers determined the genetic regulation of blood estrone levels in postmenopausal women in order to investigate links between their genetic loci and endometrial cancer. After menopause, a woman’s ovaries stop producing estrogens, resulting in extremely low or undetectable serum estradiol values. The contributing estrogen, estrone, is generated in non-ovarian tissues such as fat in Type 1 estrogen-sensitive endometrial cancer, which accounts for more than 80% of all endometrial malignancies and primarily affects menopausal women. Following the generation of estrone in these tissues, the hormone is transformed into estradiol, a stronger type of estrogen.
Previous research has found that obesity is a substantial risk factor for Type 1 endometrial cancer. This relationship may be due to a higher overall fat mass, which allows for more estrone synthesis.
Alternatively, several hereditary variables may cause a rise in estrone production after menopause, increasing the risk of endometrial cancer in these individuals. In fact, one mutation in the CYP19A1 gene, which is involved in the aromatization of testosterone to estradiol, has been linked to increased estradiol levels and postmenopausal endometrial cancer.
Importantly, investigations looking into the link between CYP19A1 polymorphisms and endometrial cancer risk have been limited due to small sample sizes and a lack of appropriate analytical tools.
About the study
In the current genome-wide association study (GWAS), researchers used liquid chromatography-tandem mass spectrometry (LCMS) to detect single nucleotide polymorphisms (SNPs) associated with sex hormone concentrations. The relationship between hormone-associated SNPs and endometrial cancer was found in 205,427 white British females aged 39 to 71, 0.9% of whom were diagnosed with the disease.
The study also included women aged 70 and up from the Sex Hormones in Older Women (SHOW) and the ASPirin in Reducing Events in the Elderly (ASPREE) trials. Non-fasting blood samples were collected from these study participants to evaluate sex hormone concentrations using LCMS.
Study findings
\The final analytic cohort included 4,951 postmenopausal women of European ethnicity, with a median age of 73.9. No genome-wide signals were found for testosterone or DHEA, which is the precursor to both estrone and testosterone. After accounting for age and BMI, the GWAS identified four distinct SNPs for estrone concentrations that were less than the genome-wide significance level.
These SNPs included rs34670419, which may be involved in transcriptional control; rs56400819, which contributes to the DNA damage response; rs2846729, which is mapped to an RNA gene; and rs2414098, which is mapped to CYP19A1. The SNP with the highest prevalence in this study cohort was rs56400819 (45%), whereas rs34670419 had the lowest prevalence (4%).
Lower estrone levels were reported for rs34670419, rs2846729, and rs2414098 carriers compared to rs56400819 carriers, who had greater estrone concentrations. After controlling for age at recruitment, BMI, parity, and diabetes history, carriers of rs2414098 had a significantly decreased risk of endometrial cancer.
Conclusions
Previous GWAS on endometrial cancer have mostly focused on estradiol concentrations; however, this hormone is frequently difficult to measure reliably, especially in postmenopausal women. In comparison, the current study evaluated estrone concentrations and found a dose-response association between the discovered SNPs and estrone concentrations.
Importantly, the researchers of the current study limited one of their analyses on rs2414098 to women over the age of 58 in order to ensure postmenopausal status. These findings demonstrate that this SNP’s effect on cancer risk is due to estrone and is unaffected by circulating estrogens and progesterone.
The recent study emphasizes the need of assessing estrone levels, as well as other sex hormones, in postmenopausal women to evaluate cancer risk. This study’s key strengths were the confirmation of postmenopausal women in the study cohort, the huge sample size, and the use of LCMS, a very sensitive and exact analytical equipment.
For more information: Genome-wide association study identifies genetic regulation of oestrone concentrations and association with endometrial cancer risk in postmenopausal women, eBioMedicine, doi:10.1016/j.ebiom.2024.104997
more recommended stories
 New Blood Cancer Model Unveils Drug Resistance
New Blood Cancer Model Unveils Drug ResistanceNew Lab Model Reveals Gene Mutation.
 Healthy Habits Slash Diverticulitis Risk in Half: Clinical Insights
Healthy Habits Slash Diverticulitis Risk in Half: Clinical InsightsHealthy Habits Slash Diverticulitis Risk in.
 Caffeine and SIDS: A New Prevention Theory
Caffeine and SIDS: A New Prevention TheoryFor the first time in decades,.
 Microbial Metabolites Reveal Health Insights
Microbial Metabolites Reveal Health InsightsThe human body is not just.
 Reelin and Cocaine Addiction: A Breakthrough Study
Reelin and Cocaine Addiction: A Breakthrough StudyA groundbreaking study from the University.
 Preeclampsia and Stroke Risk: Long-Term Effects
Preeclampsia and Stroke Risk: Long-Term EffectsPreeclampsia (PE) – a hypertensive disorder.
 Statins and Depression: No Added Benefit
Statins and Depression: No Added BenefitWhat Are Statins Used For? Statins.
 Azithromycin Resistance Rises After Mass Treatment
Azithromycin Resistance Rises After Mass TreatmentMass drug administration (MDA) of azithromycin.
 Generative AI in Health Campaigns: A Game-Changer
Generative AI in Health Campaigns: A Game-ChangerMass media campaigns have long been.
 Molecular Stress in Aging Neurons Explained
Molecular Stress in Aging Neurons ExplainedAs the population ages, scientists are.

Leave a Comment